Traditional Chinese medicine monomer composition for antagonizing lung injury and its application
A monomer composition and lung injury technology, applied in the direction of drug combination, pharmaceutical formula, antiviral agent, etc., can solve the problems of severe infection of lung tissue, decreased phagocytosis, aggravation of lung tissue damage, etc., to achieve inhibition of pulmonary fibrosis, Inhibit EMT and promote cell repair effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
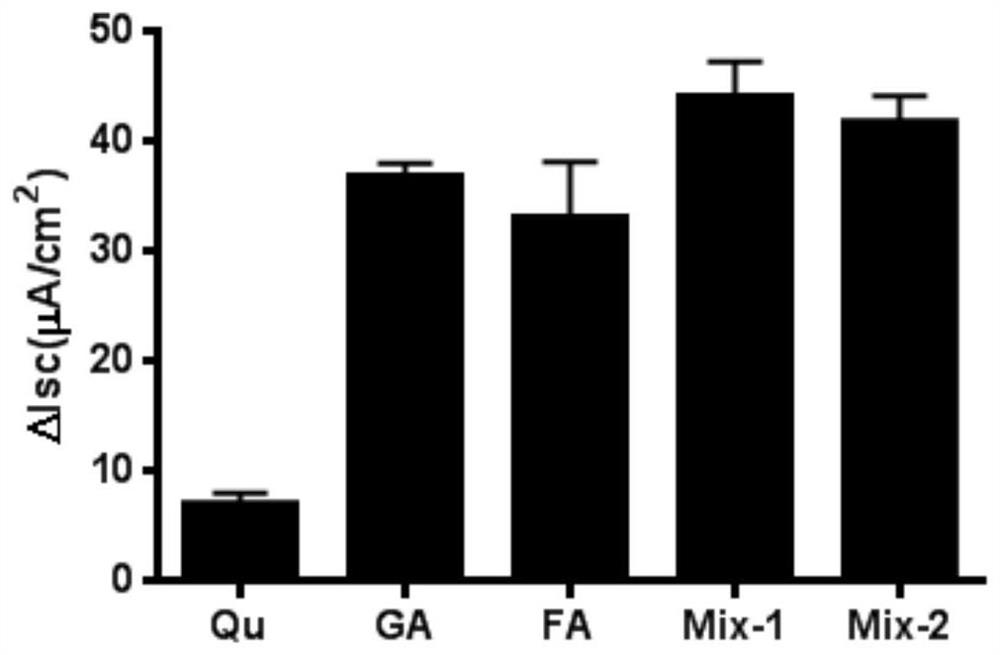
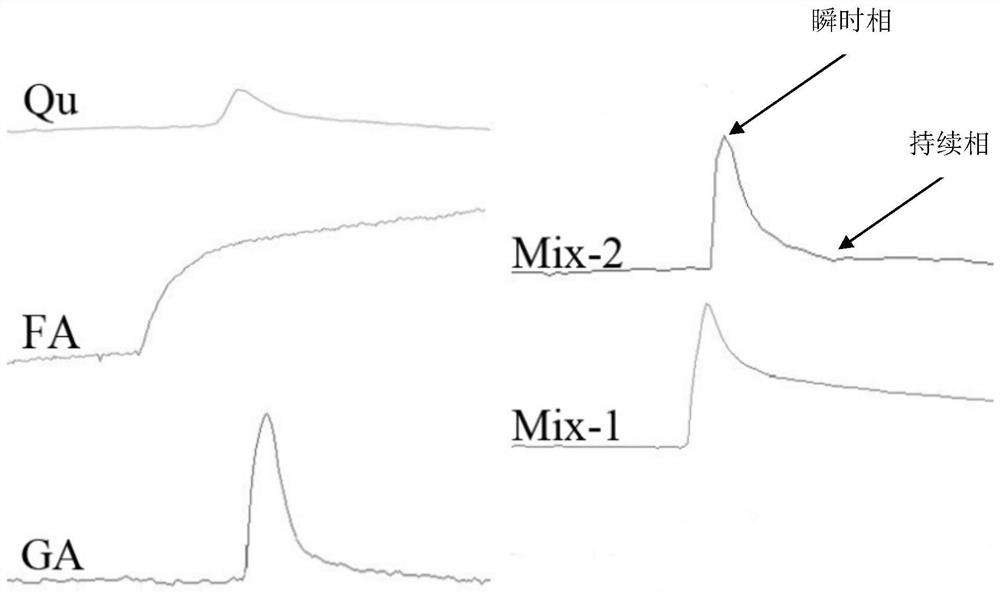
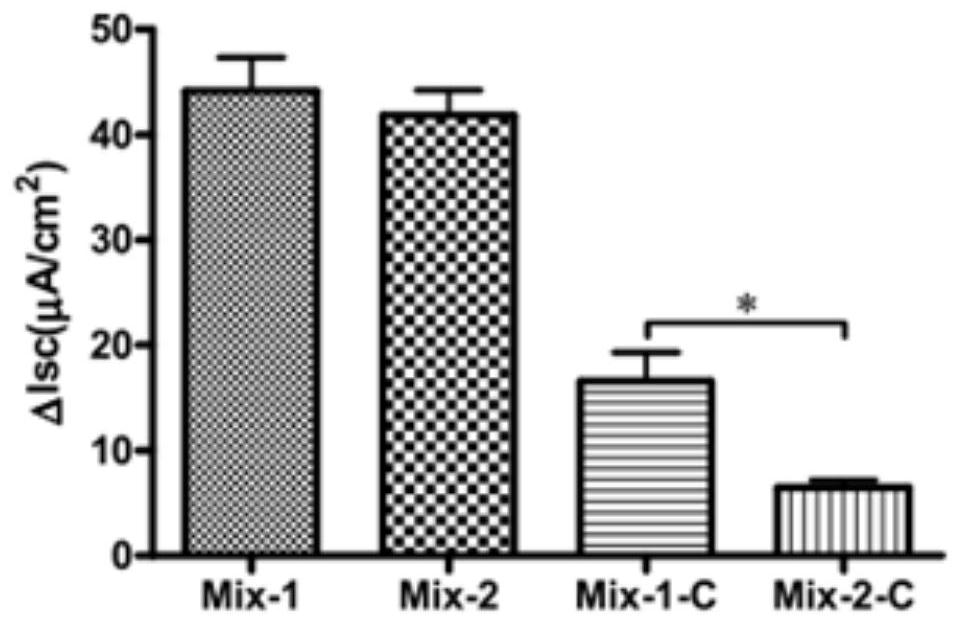
[0057] The molar ratio between quercetin, glycyrrhizic acid and ferulic acid is 1:1:1, that is, the molar concentration of ferulic acid is 100 μM, the molar concentration of glycyrrhizic acid is 100 μM, and the molar concentration of quercetin is 100 μM. It is prepared into tablets with a pharmaceutically acceptable carrier through a conventional process.
Embodiment 2
[0059] The molar ratio between quercetin, glycyrrhizic acid and ferulic acid is 1:2:1. That is, the molar concentration of ferulic acid is 200 μM, the molar concentration of glycyrrhizic acid is 100 μM, and the molar concentration of quercetin is 100 μM. Prepare capsules with pharmaceutically acceptable carriers through conventional techniques.
Embodiment 3
[0061] The molar ratio between quercetin, glycyrrhizic acid and ferulic acid is 1:3:1. That is, the molar concentration of ferulic acid is 300 μM, the molar concentration of glycyrrhizic acid is 100 μM, and the molar concentration of quercetin is 100 μM. Prepared into microcapsules with pharmaceutically acceptable carriers through conventional techniques.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com