Sensor for detecting 8-hydroxyl-2'-deoxyguanosine as well as synthesis method and application thereof
A deoxyguanosine and sensor technology, applied in the fields of sensor synthesis and photochemical analysis, can solve problems such as limited scientific knowledge, and achieve huge market benefits, great application value, and simple preparation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
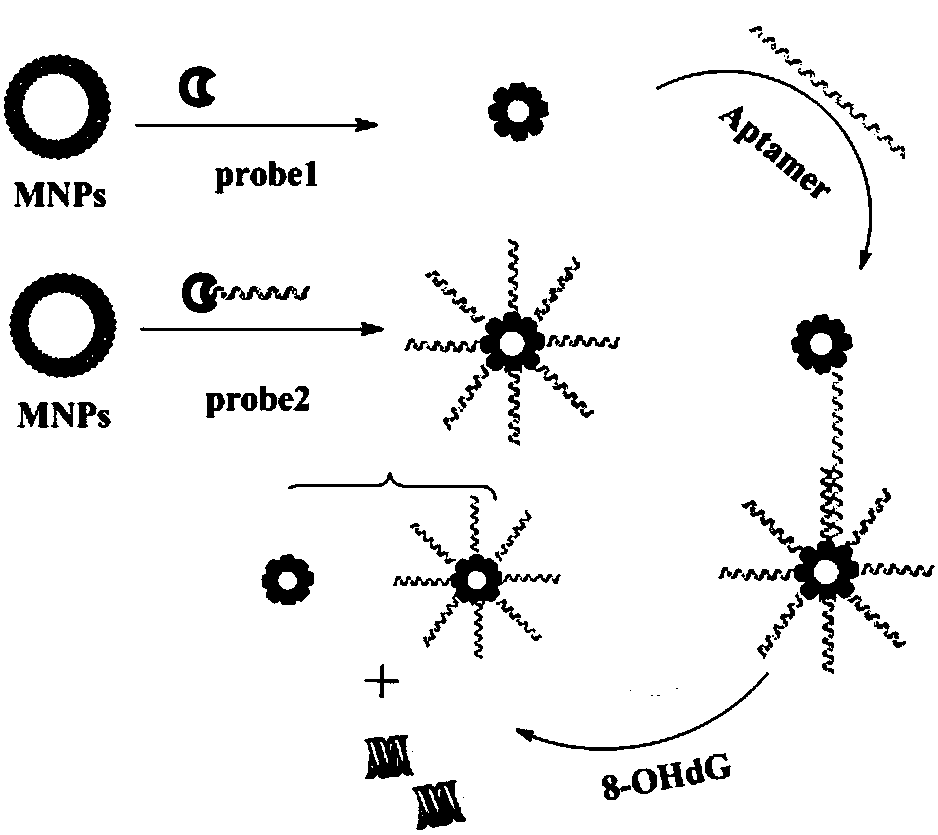
[0058] Synthetic method of 8-hydroxy-2'-deoxyguanosine sensor sensor
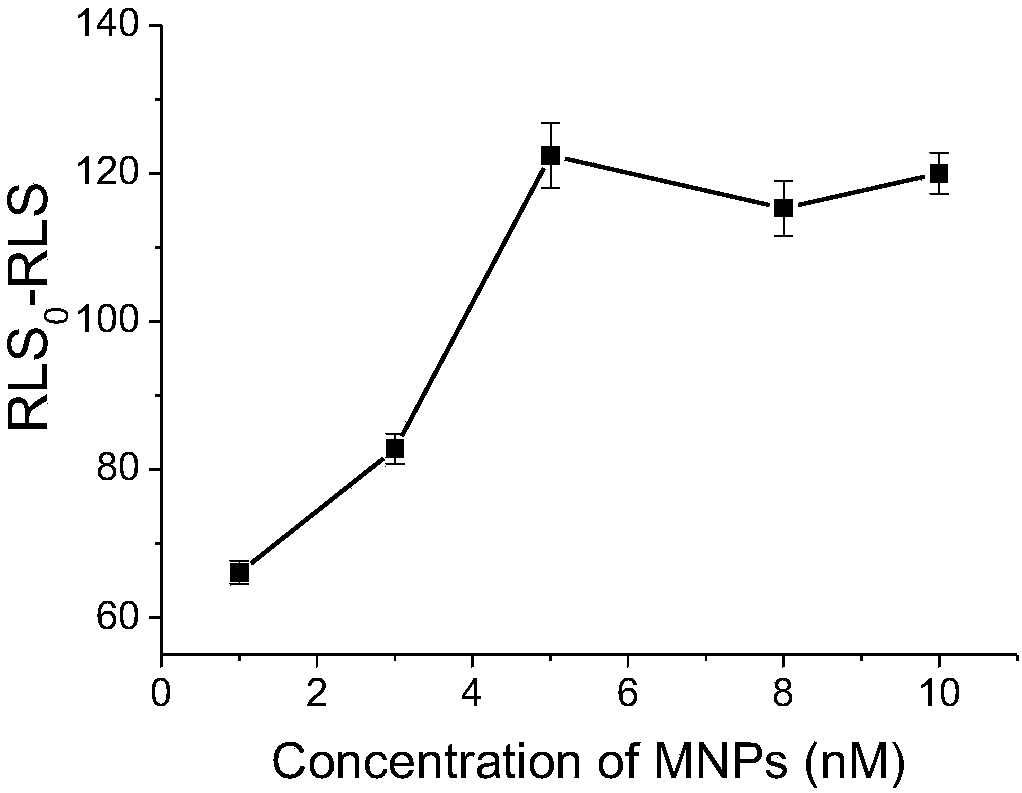
[0059] Two copies of 5 μL (microliter) of magnetic nanoparticles having a mass concentration of 1 mg / mL that have been modified with streptavidin were taken respectively, and a deoxynucleotide sequence chain ( probe1 and probe2) 7.5 μL (microliter), then dilute the two solutions into 1 mL of buffer solution, and react at 30°C for 20 minutes; after the reaction, mix the two solutions evenly, and then add 7.5 μL of a deoxynucleotide sequence (aptamer) solution with a molar concentration of 100nM / mL that can specifically bind to 8-hydroxy-2'-deoxyguanosine, and react at 30°C for 20 minutes, so that the 8-hydroxy - 2'-deoxyguanosine sensor.
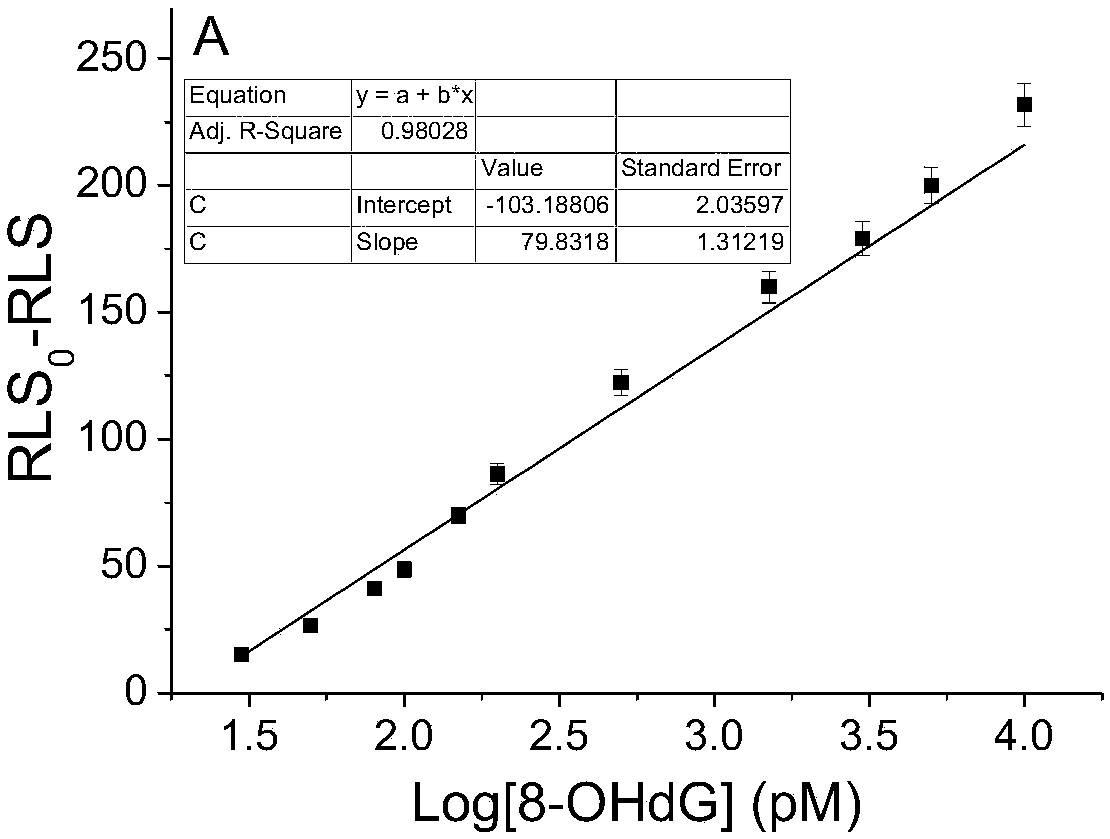
[0060] figure 2 It can be seen that when the concentration of 8-hydroxy-2'-deoxyguanosine reaches pM (linear range 30pM–10.0nM), the reaction is extremely sensitive. Since the change value of the resonance light scattering signal is inversely proportional to the molecu...
Embodiment 2
[0208] Using the 8-hydroxy-2'-deoxyguanosine sensor prepared in Example 1. 3.0nM 8-hydroxy-2'-deoxyguanosine, the concentration is 100nM / L creatinine (creatinine), bovine serum albumin (BSA), ascorbic acid (ascorbic acid), sodium oxalate (disodium oxalate), sucrose (sucrose) , urea, uric acid, glucose, 2'-deoxyguanosine, ganciclovir, β-thymidine, guanine ), Mg 2+ 、K + , Ca 2+ , Fe 3+ 、Cu 2+ Test the resonant light scattering signal respectively.
[0209] Figure 8 Shown is a synthetic 8-hydroxy-2'-deoxyguanosine sensor that detects 8-hydroxy-2'-deoxyguanosine even when other biomolecules are present at higher concentrations than 8-hydroxy-2'-deoxyguanosine , still will not affect the detection of 8-hydroxy-2'-deoxyguanosine. So the 8-hydroxy-2'-deoxyguanosine sensor has good selectivity to 8-hydroxy-2'-deoxyguanosine.
Embodiment 3
[0211] Sensor reuse
[0212] After the 8-hydroxy-2'-deoxyguanosine sensor prepared in Example 1 was placed on the magnetic separator for 24 hours, the magnetic microspheres were tightly attracted by the magnetic separator. Carefully remove the supernatant, add a solution of a deoxynucleotide sequence (aptamer) that can specifically bind to 8-hydroxy-2'-deoxyguanosine, so that the concentration is 0.75nmol / mL, and finally dilute the whole system into 2mL of buffer solution, shake well and react at 30°C for 20 minutes, thus assembling a reusable 8-hydroxy-2'-deoxyguanosine sensor. By analogy, the same situation, the sensor can be reused.
[0213] Figure 9 The cycle is shown, and the high and low points on each broken line represent the resonance light scattering intensity before and after adding 8-hydroxy-2'-deoxyguanosine. It shows that the sensor performs well in repeated use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com