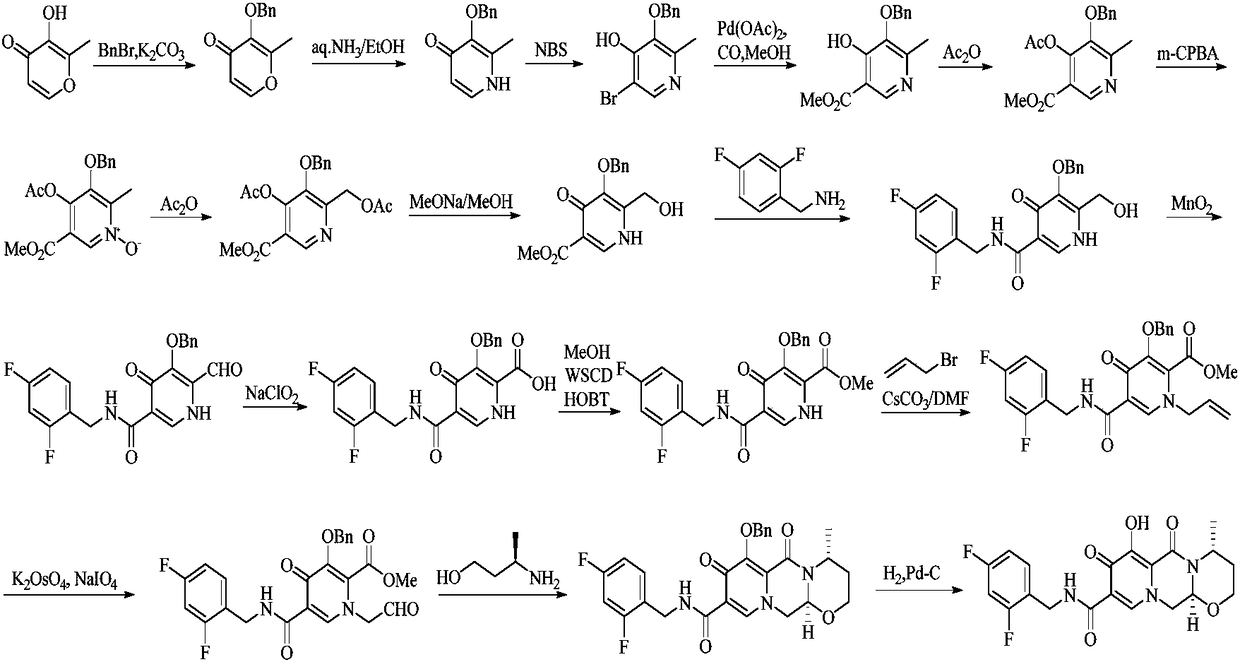
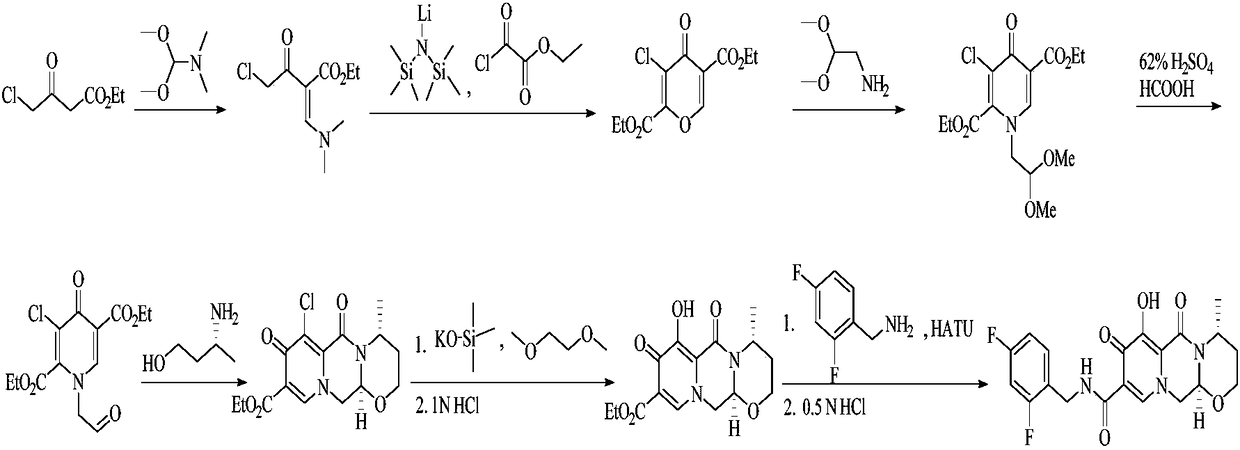
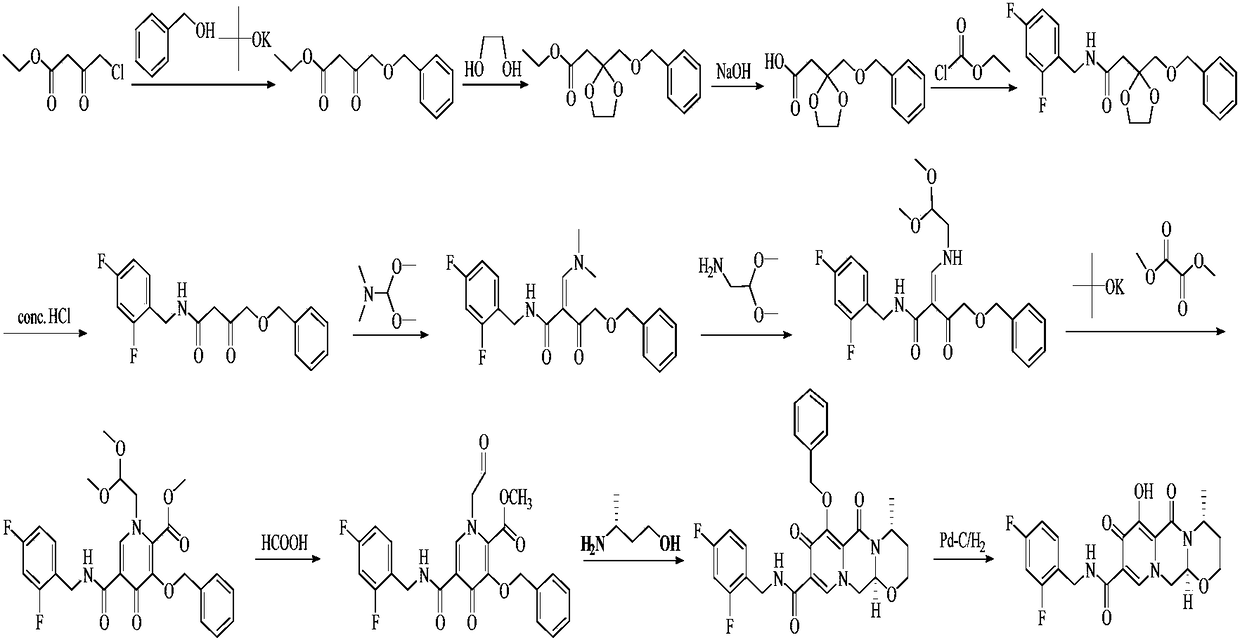
Improved method for synthesizing dolutegravir
A synthesis method and mixed solvent technology, applied in the synthesis field of dolutegravir, can solve the problems of low conversion rate of raw materials, complex post-treatment, long reaction route, etc., and achieve high purity, high yield and total yield, and product The effect of stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Add 100 g of compound 1, 132 g of potassium carbonate and 105 g of benzyl chloride into 300 mL of methanol, and heat to reflux for 1 hour to complete the reaction. Cool the system to room temperature, filter, concentrate the filtrate, dissolve the residue in 200 mL of dichloromethane, wash with 5% sodium hydroxide solution (50 mL×2) and saturated sodium chloride solution (50 mL) successively, dry the organic phase, and concentrate Obtain 168g of yellow oily substance, i.e. intermediate 2, yield: 98%.
[0034] (2) Add 50g of periodic acid into 350mL of acetonitrile, stir and dissolve, then add 0.2g of chromium trioxide and 20g of intermediate 2 to the system in turn, stir at room temperature for 1.5 hours, and the reaction ends. The solvent was evaporated to dryness, and 100 mL of dichloromethane and 200 mL of water were added to the residue solution. After the residue was completely dissolved, the organic phase was separated, and the aqueous phase was extracted with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com