New methods for making barusiban and its intermediates
An intermediate, homocysteine technology, which is applied in the new field of manufacturing balusaban and its intermediates, and can solve the problems of cumbersome large-scale manufacturing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0256] Example 1 : Synthesis of Parucipan
[0257] 1.1. Synthesis flow chart
[0258]
[0259]
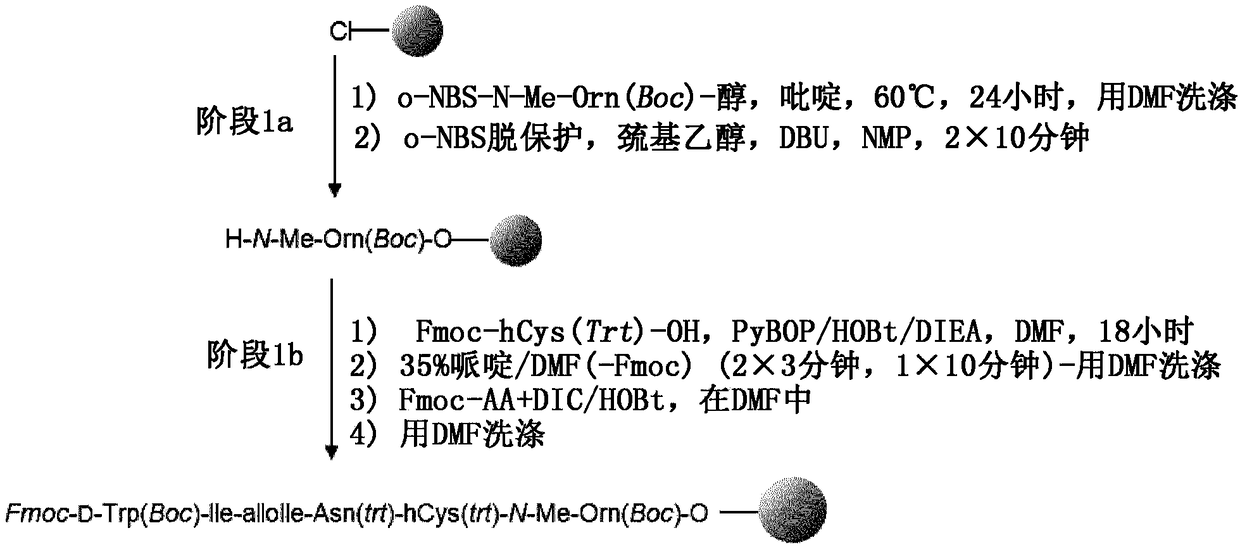
[0260] 1.2. Description of the manufacturing method
[0261] to assemble
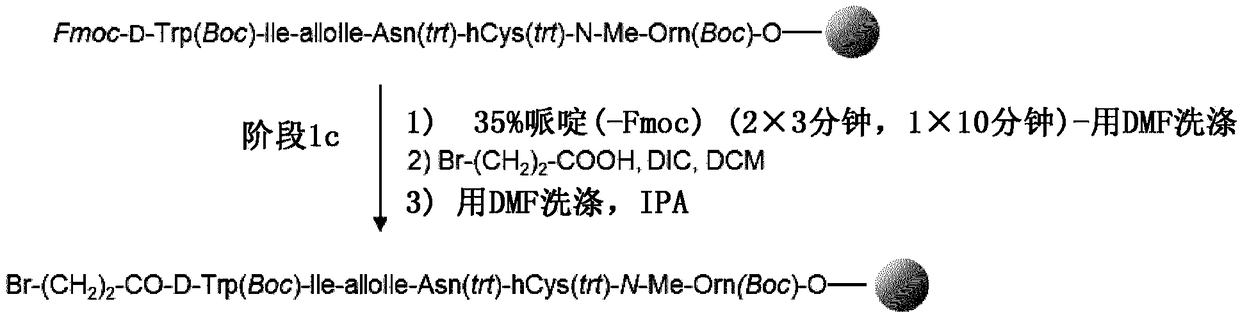
[0262] Direct coupling of protected o-NBS-N-MeOrn(Boc)-alcohol to chloro-2-chlorotrityl resin (CTC resin) in DMF in the presence of pyridine at 60 °C within 17 hours . After capping the resin, the NBS group was deprotected by washing with a DBU / mercaptoethanol / NMP mixture. The second protected amino acid (Fmoc-hCy(Trt)-OH) was coupled with PyBOP / HOBt / DIEA in DMF after removal of the NBS protecting group. Reaction completion was checked by coupling test (Chloranil test). Four additional residues (Fmoc-Asn(Trt)-OH, Fmoc-AlloIle-OH, Fmoc-Ile-OH and Fmoc-D-Trp(Boc)-OH) were introduced by successive cycles of Fmoc deprotection and amino acid coupling ):
[0263] 1. Fmoc removal
[0264] 2. DMF washing
[0265] 3. Fmoc-AA-OH will be coupled with DIC / HOBt in DMF.
[0266] 4. Conjugation test (ninh...
Embodiment 2
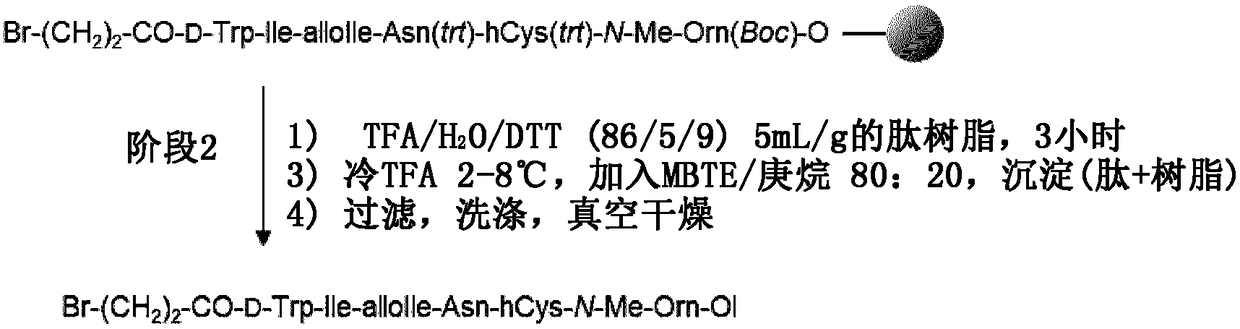
[0273] 2. Example 2: Using 3-chloropropionic acid instead of 3-bromopropionic acid to synthesize crude balucipan
[0274] The experiment was carried out according to the method described above (see Example 1), wherein 3-chloropropionic acid was used instead of 3-bromopropionic acid.
[0275] 2.1. Assembly
[0276] resin loading
[0277] The assembly was performed on a 5 millimolar scale (7.14 g chloro-2-chlorotrityl resin (CTC resin) with a degree of substitution of 0.7 meq / g). After swelling the resin in DMF (7 mL) within 15 minutes, o-NBS-N-MeOrn(Boc)-alcohol (1 equiv, 2.92 g) was dissolved in 9 mL DMF and added onto the resin. Pyridine (2 equiv, 0.81 mL) was added and the reaction mixture was heated to 60 °C and stirred over 17 hours. After 1 hour, 6 mL of DMF was added to homogenize the reaction mixture. The concentration of o-NBS-N-MeOrn(Boc)-alcohol during the loading reaction was 0.22 mol / L. After 17 hours, 15 mL of DMF was added to the resin to homogenize the re...
Embodiment 3
[0311] 3. Example 3. According to different synthetic pathways for the synthesis of balucipan
[0312] 3.1. Synthesis flow chart
[0313] A flow chart of the production of balusiaban according to the method of Example 3 is shown below:
[0314]
[0315]
[0316] 3.2. Description of the manufacturing method
[0317] 3.2.1. Assembly
[0318] The protected Fmoc-N-MeOrn(Boc)-alcohol was directly coupled to the carboxyl resin in DMF in the presence of DMPA within 2 hours at room temperature. After capping the resin, the Fmoc group was deprotected by washing with piperidine solution (20% in DMF). Five additional residues (Fmoc-hCy(mmt)-OH, Fmoc-Asn(Trt)-OH, Fmoc-alloIle-OH, Fmoc-Ile-OH and Fmoc- D-Trp(Boc)-OH):
[0319] 1. Fmoc removal
[0320] 2. DMF washing
[0321] 3. Coupling of Fmoc-AA-OH with DIC / HOBt in DMF by Preactivating Amino Acids
[0322] 4. Coupling test (ninhydrin test or chloranil test)
[0323] 5. DMF washing
[0324] Reaction volumes were calculated...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com