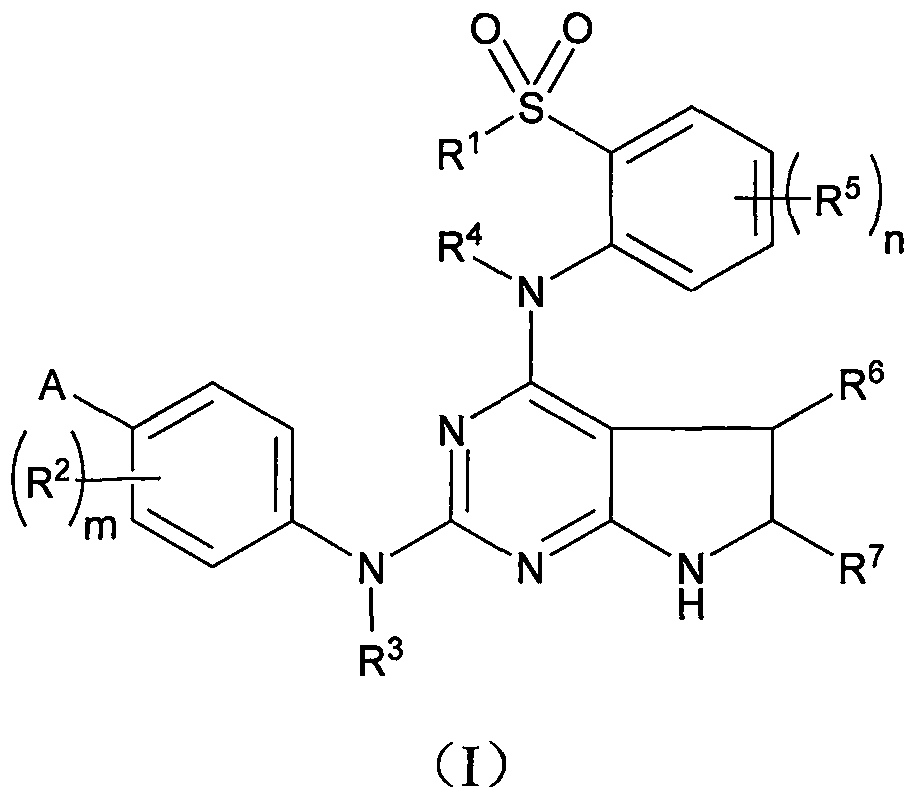
Anaplastic lymphoma kinase (ALK) inhibitor, and preparation method and application thereof
A technology for selecting compounds, used in antitumor drugs, pharmaceutical formulations, organic active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0070] The compounds of the present invention and their preparation methods and uses are illustrated below in conjunction with the examples.
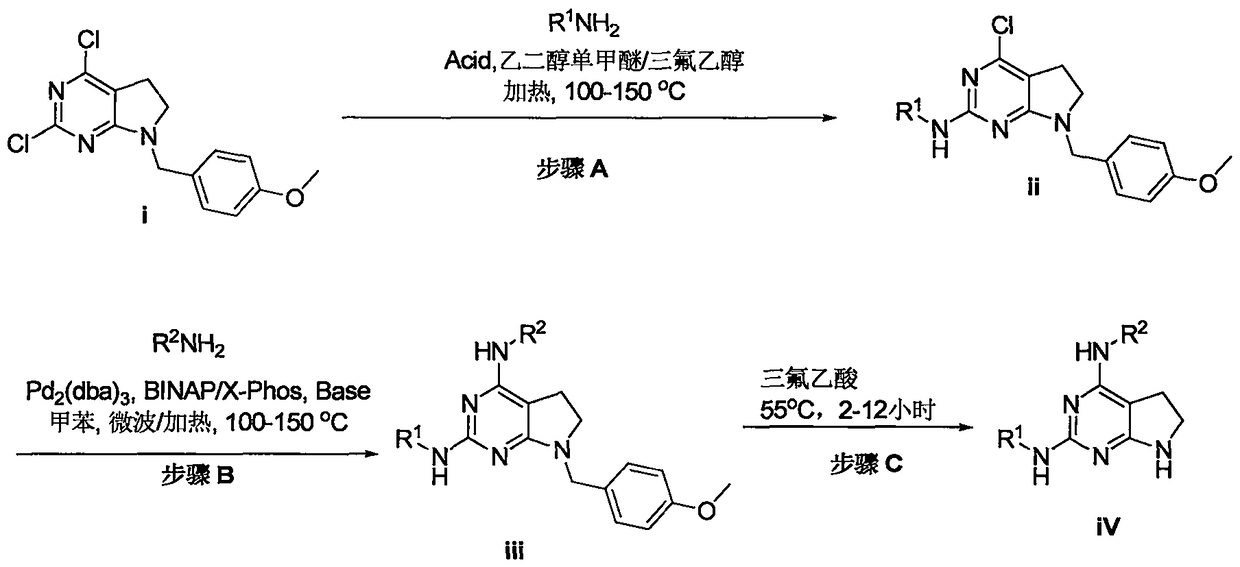
[0071] Process 1.:
[0072]
[0073] The compounds shown in the present invention can be prepared according to the route described in Scheme 1. The product obtained from the reaction in scheme 1 can be obtained by conventional separation techniques, such traditional techniques include but not limited to filtration, distillation, crystallization, chromatographic separation and the like. Starting materials can be synthesized in-house or purchased from commercial establishments such as, but not limited to, Adrich or Sigma. These starting materials can be characterized using conventional means, such as physical constants and spectral data. The compounds described in this invention may be obtained using synthetic methods as single isomers or as mixtures of isomers.
[0074] Step A: 2,4-dichloro-7-(4-methoxybenzyl)-6,7-dihydro-5H-pyrrol...
Embodiment 1
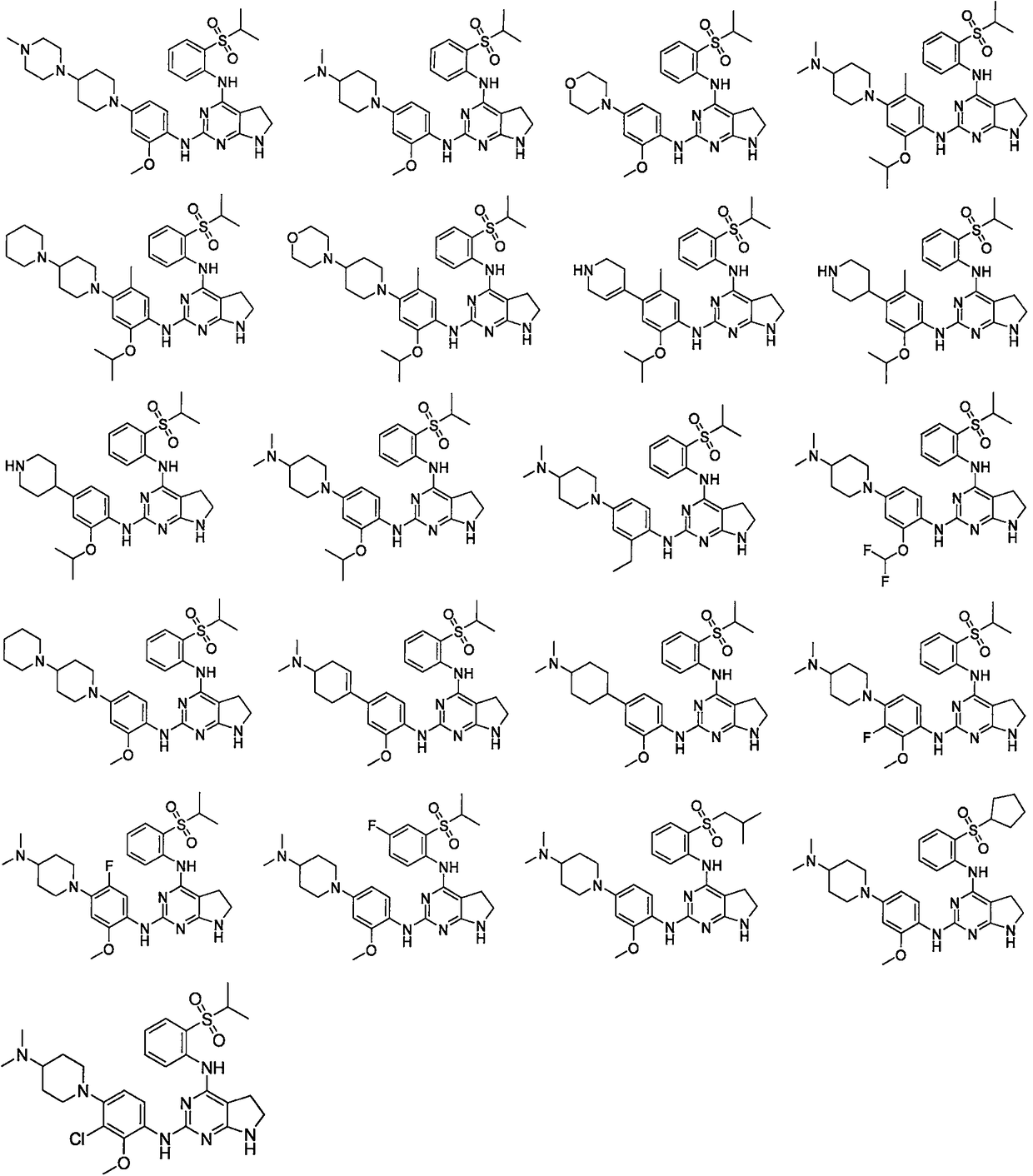
[0223] Example 1: N2-(4-(4-(dimethylamino)piperidin-1-yl)-2-methoxyphenyl)-N4-(2-(isopropylsulfonyl Acyl)phenyl)-6,7-dihydro-5H-pyrrolo[2,3-d]pyrimidine-2,4-diamine
[0224]
[0225] Step 1: 4-Chloro-N-(4-(4-(dimethylamino)piperidin-1-yl)-2-methoxyphenyl)-7-(4-methoxybenzyl)- 6,7-Dihydro-5H-pyrrolo[2,3-d]pyrimidin-2-amine
[0226]
[0227] According to general synthetic method A, 2,4-dichloro-7-(4-methoxybenzyl)-6,7-dihydro-5H-pyrrolo[2,3-d]pyrimidine (intermediate i , 680 mg), 1-(4-amino-3-methoxyphenyl)-N, N-dimethylpiperidin-4-amine (603 mg) and trifluoroacetic acid (0.16 ml, 250 mg) followed by Added to 40 ml of ethylene glycol monomethyl ether: trifluoroethanol (2:1, v / v) mixed solvent, heated at 120°C to reflux for 24h, the reaction solution was concentrated under reduced pressure, and the obtained residue was washed with a small amount of dichloro After methane and water were dissolved, adjusted to pH = 8 with saturated sodium bicarbonate solution, extracted...
Embodiment 2
[0233] Example 2: N4-(2-(isopropylsulfonyl)phenyl)-N2-(2-methoxy-4-(4-(4-methylpiperazin-1-yl) piperidin-1-yl)phenyl)-6,7-dihydro-5H-pyrrolo[2,3-d]pyrimidine-2,4-diamine
[0234]
[0235] The target compound was obtained by the same procedure as in Example 1 using different reactants. 1 H-NMR (400MHz, DMSO-d 6 ): δ=8.53-8.50 (2H, m), 7.70 (2H, dt, J 1 =6.8,J 2 =8.3Hz), 7.59-7.55(1H, m), 7.27(1H, s), 7.14(1H, d, J=7.2Hz), 6.77(1H, s), 6.60(1H, d, J=2.4Hz ), 6.43 (1H, dd, J 1 =2.4,J 2 =8.7Hz), 3.79(3H, s), 3.66(2H, d, J=11.8Hz), 3.53(2H, t, J=8.5Hz), 3.40(1H, sept, J=6.8Hz), 2.80- 2.76(2H, m), 2.64-2.59(2H, m), 2.56-2.51(2H, m), 2.39-2.28(5H, m), 2.15(3H, s), 1.84(2H, d, J=12Hz ), 1.51 (2H, dq, J 1 =3.2,J 2 = 12 Hz), 1.23 (2H br s), 1.17 (6H, d, J = 6.8 Hz). ESI MS m / z: 621.28[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com