Applications of CREG in treatment of nonalcoholic fatty liver disease and type 2 diabetes
A type 2 diabetes and fatty liver technology, applied in the field of CREG preparations, can solve the problems of gene regulation and therapeutic preparations for untreated diabetes, and achieve the effect of improving fatty liver
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Embodiment 1. mouse fatty liver, type 2 diabetes model (diet induced obesity, DIO) preparation
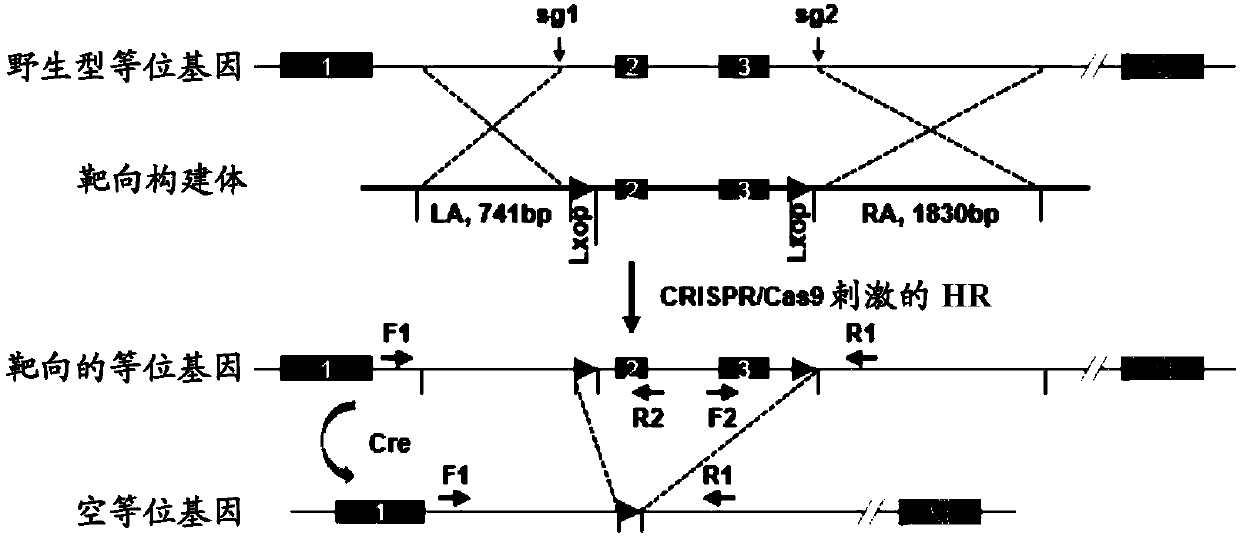
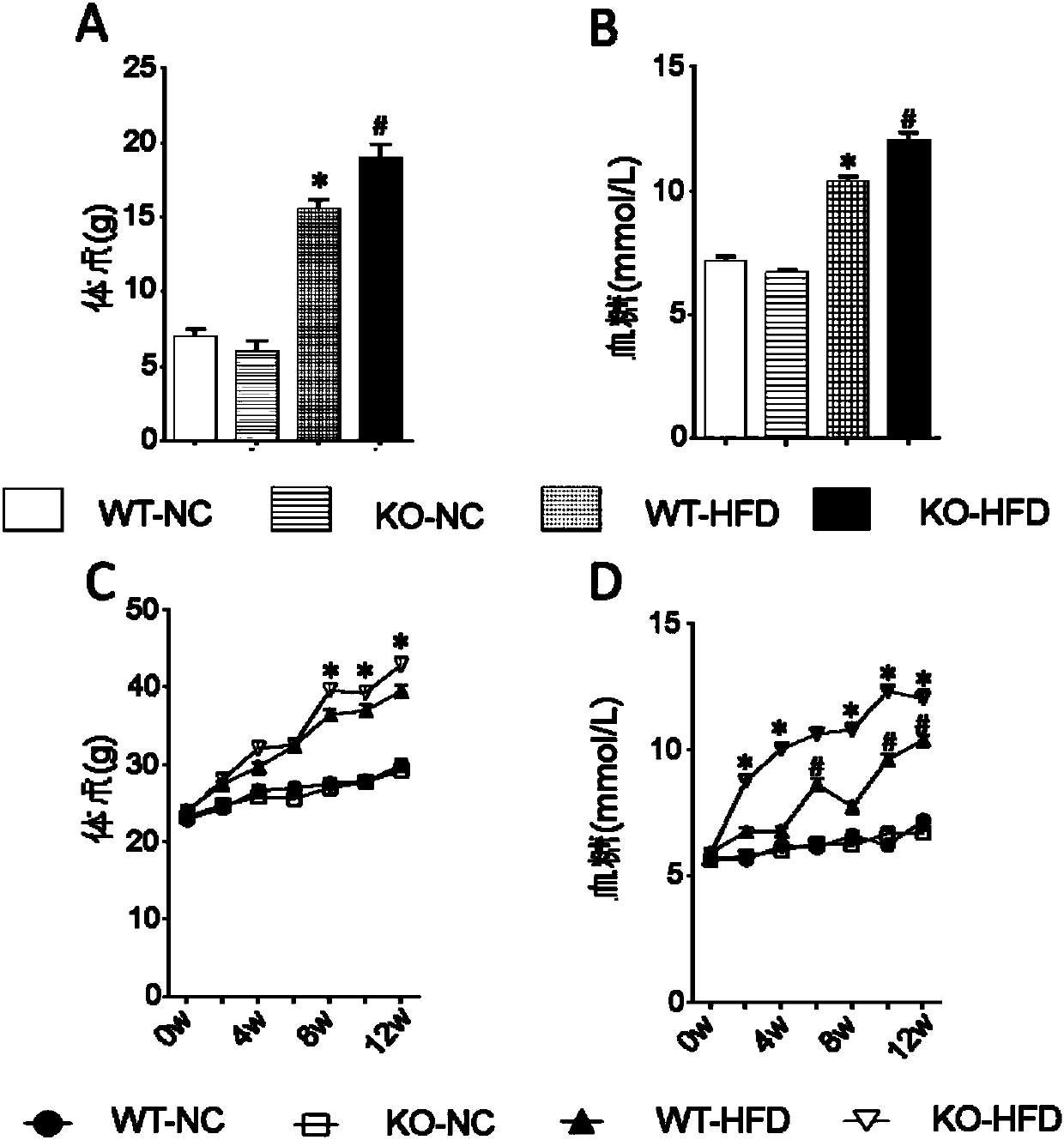
[0069](1) Grouping of animals: 8-week-old male WT mice and CREG-KO (or CREG-TG) mice obtained by the aforementioned method were selected and given two special feeds, D12942 high fat diet (High fat diet, HFD) and D12450B Normal chow (NC) feeding, that is, WT-NC group, KO-NC group, WT-HFD group, KO-HFD group, a total of 4 groups (or WT-NC group, TG-NC group, WT-HFD group group, TG-HFD group).
[0070] (2) Operation procedure for the preparation of DIO mice:
[0071] Phenotype correlation analysis was performed on WT and KO (TG) mice to clarify the role of CREG gene on fatty liver and type 2 diabetes. Eight-week-old, male, WT mice and CREG-KO (TG) mice were selected and fed with two special diets, D12942 high-fat diet (Highfat diet, HFD) and D12450B low-fat diet (Normal chow, NC), respectively, namely There are 4 groups including WT-NC group, KO / TG-NC group, WT / TG-HFD group ...
Embodiment 2
[0072] Embodiment 2. Determination of mouse body weight and blood sugar level
[0073] (1) Detection of fasting body weight and feed amount of mice
[0074] ①Fasting: fast the mice to be tested at 8:00 am (without water), and start the experimental operation at 2:00 pm.
[0075] ② Weighing: Weigh at the 0th week, 4th week, 8th week, and 12th week respectively, put a small plastic bucket on the dynamic electronic balance, grab the mouse, put it into the weighing bucket, measure the body weight and record the data .
[0076] ③ Feed amount detection: After the weighing operation is completed, add feed to the mice, and record the amount of feed for the mice on the dynamic electronic balance.
[0077] (2) Detection of fasting blood glucose level in mice
[0078] All the mice to be tested were fasted from 8:00 am to 2:00 pm (without water), that is, the experimental operation was started after 6 hours of fasting.
[0079] ① Blood glucose meter preparation: Check the battery of t...
Embodiment 3
[0084] Example 3. Mouse glucose tolerance test (intraperitoneal glucose tolerance test, IPGTT)
[0085] In the twelfth week of the experiment, the intraperitoneal injection of glucose test (IPGTT) was performed to evaluate the ability of the mice to tolerate glucose.
[0086] (1) Before measuring blood glucose, measure the fasting body weight of the mice, and calculate the injection volume of glucose based on 10 μL / g.
[0087] (2) First detect the fasting blood glucose at 0 minutes before the glucose injection, and inject the glucose solution intraperitoneally quickly after the detection is completed.
[0088] (3) Operation method of intraperitoneal injection
[0089] ①Fix the mouse; hold the mouse, the little finger and ring finger of the left hand grasp the mouse's tail, and the other three fingers grasp the mouse's neck, so that the mouse's head is down and the mouse's abdomen is fully exposed.
[0090] ②Needle positioning and injection: insert the needle from the side of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com