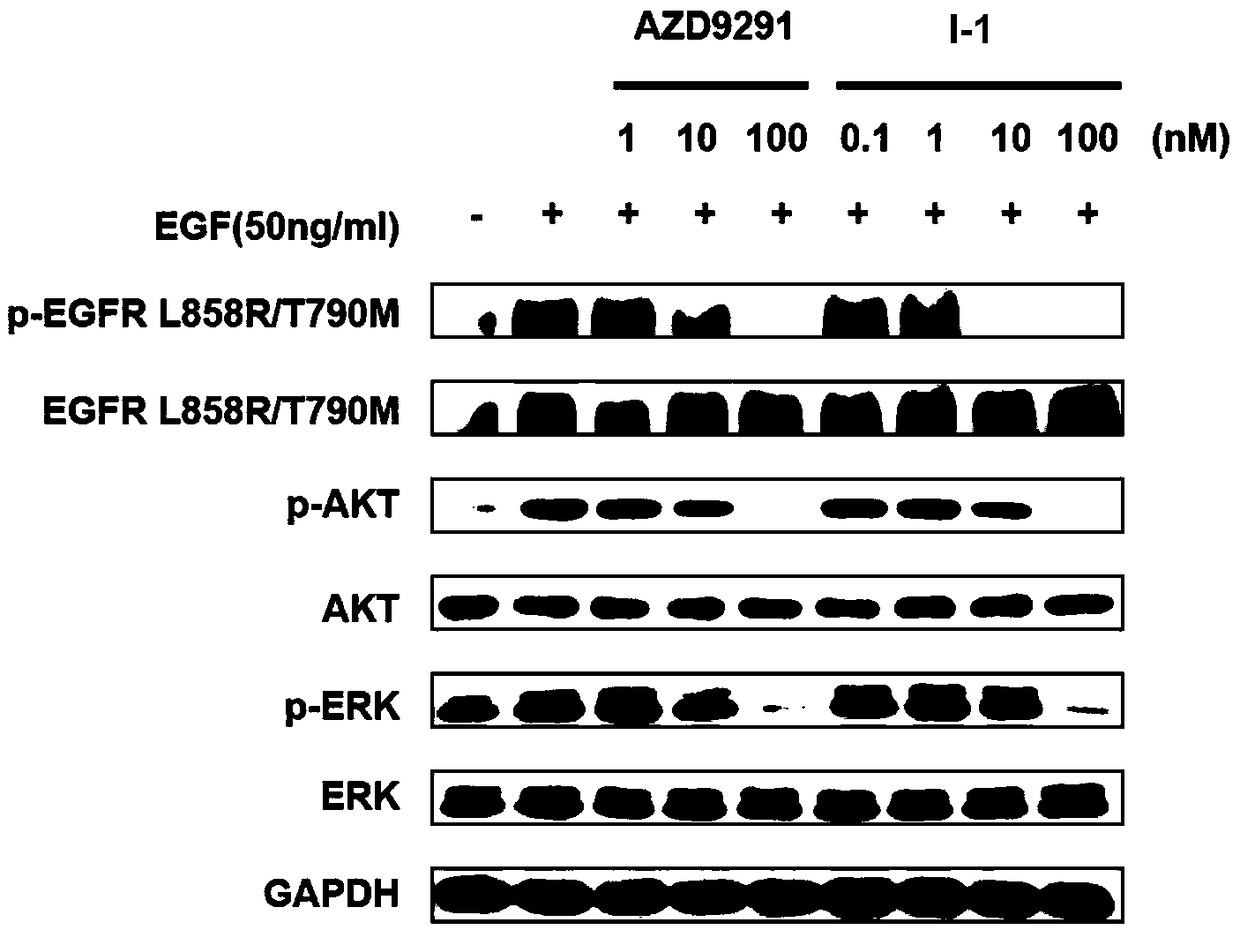
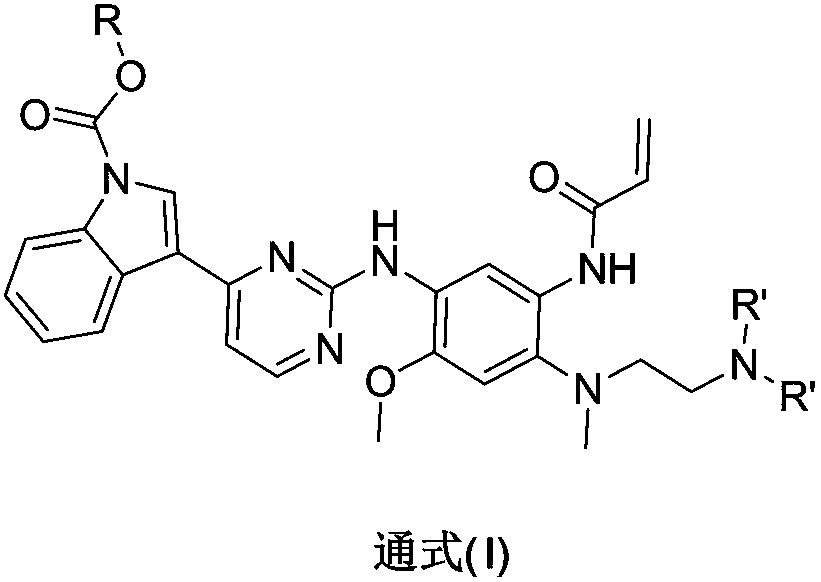
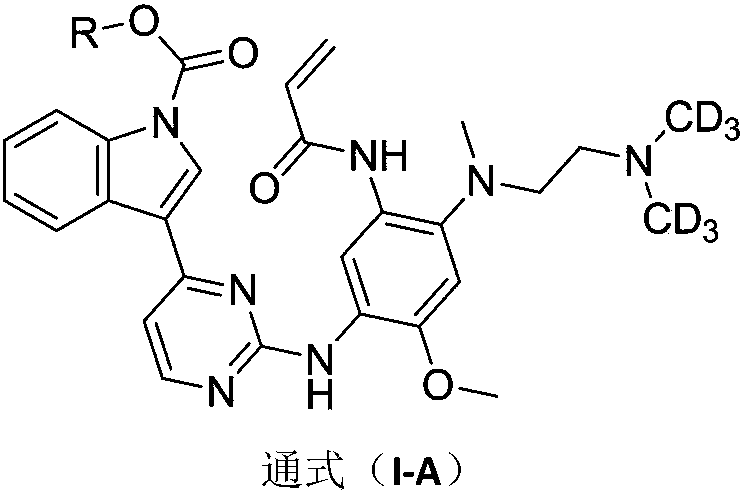
Indole-1-carbonate compound and preparation method and application thereof
A technology of carbonates and compounds, applied in the field of drug synthesis, can solve problems such as poor oral exposure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] 2-(2-((5-acrylamide-4-((2-(dimethylamino)ethyl)(methyl)amino)-2-methoxyphenyl)amino)pyrimidin-4-yl) -1H-indole-1-methyl carbonate (I-1)
[0102] Synthesis method 1:
[0103] N-(5-((4-(1H-indol-3-yl)pyrimidin-2-yl)amino)-2-((2-(dimethylamino)ethyl)(methyl)amino) -4-Methoxyphenyl)acrylamide (ie AZ5104, 3.0g, 6.18mmol) was dissolved in 200mL of anhydrous tetrahydrofuran, and sodium hydride (packed in mineral oil, 60%, 740mg, 18.53mmol) was added slowly under ice-bath conditions , after stirring for 2min, methyl chloroformate (1.75g, 18.53mmol) dissolved in 50mL of tetrahydrofuran was slowly added dropwise, the ice bath was removed, and after 10min, the reaction solution was slowly poured into 200mL of saturated ammonium chloride solution, and 200mL of ethyl acetate was added to extract the organic phase, washed with saturated sodium chloride, dried over anhydrous sodium sulfate, spin-dried solvent, and purified by column chromatography to obtain 2-(2-((5-acrylamide-4-((2...
Embodiment 2
[0111] 2-(2-((5-acrylamide-4-((2-(dimethylamino)ethyl)(methyl)amino)-2-methoxyphenyl)amino)pyrimidin-4-yl) -1H-Indole-1-ethyl carbonate (I-2)
[0112] Except that methyl chloroformate is replaced by ethyl chloroformate, the synthetic method is the same as synthetic method 1 in Example 1.
[0113] 1 H NMR (400MHz, CDCl 3 )δ10.08(s,1H),9.64(s,1H),8.53(d,J=5.2Hz,1H),8.42(s,1H),8.38(d,J=7.5Hz,1H),8.26( d,J=8.1Hz,1H),7.64(s,1H),7.37(ddd,J=15.1,14.0,6.7Hz,2H),7.14(d,J=5.2Hz,1H),6.81(s,1H ),6.41(d,J=2.8Hz,2H),5.72–5.64(m,1H),4.53(q,J=7.1Hz,2H),3.90(s,3H),2.94–2.86(m,2H) , 2.72 (s, 4H), 2.36 (s, 2H), 2.31 (s, 8H), 1.49 (d, J=7.1Hz, 3H).
[0114] LR-Mass (ESI): 558.8 (M+1, C 30 h 36 N 7 o 4 ).
Embodiment 3
[0116] 2-(2-((5-acrylamide-4-((2-(dimethylamino)ethyl)(methyl)amino)-2-methoxyphenyl)amino)pyrimidin-4-yl) -1H-Indole-1-propyl carbonate (I-3)
[0117] Except that methyl chloroformate is replaced by propyl chloroformate, the synthetic method is the same as synthetic method 1 in Example 1.
[0118] 1 H NMR (500MHz, CDCl 3 )δ9.91(s,1H),9.50(s,1H),8.73(d,J=14.8Hz,1H),8.55(dd,J=14.8,3.1Hz,1H),8.11(dd,J=14.9 ,3.1Hz,1H),7.43–7.06(m,4H),6.43(s,1H),6.17(ddd,J=26.6,24.4,12.2Hz,2H),5.69(dd,J=33.3,4.6Hz, 1H), 4.69(s, 1H), 4.21(t, J=14.7Hz, 2H), 3.86(s, 3H), 3.53(dt, J=21.9, 14.6Hz, 2H), 2.75(s, 3H), 2.50 (t, J = 14.6Hz, 2H), 2.21 (s, 6H), 1.91–1.49 (m, 2H), 1.01 (t, J = 13.4Hz, 3H).
[0119] LR-Mass (ESI): 572.8 (M+1, C 31 h 38 N 7 o 4 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com