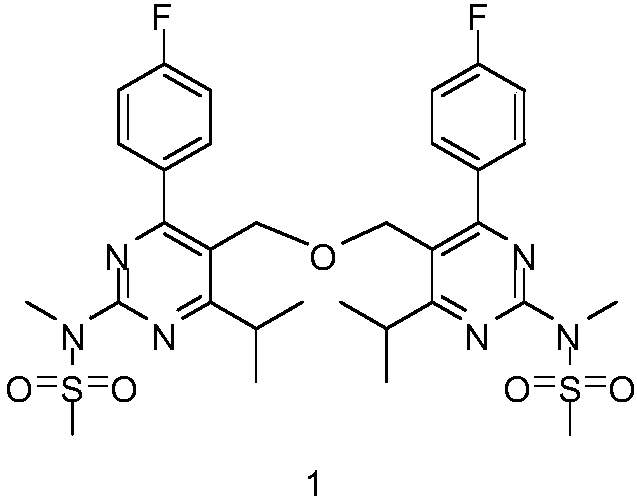
Method for synthesizing rosuvastatin calcium intermediate impurity
A technology of rosuvastatin calcium and a synthesis method, which is applied in the field of chemical pharmacy to achieve the effects of strengthening control, cheap raw materials, and improving accurate positioning and characterization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
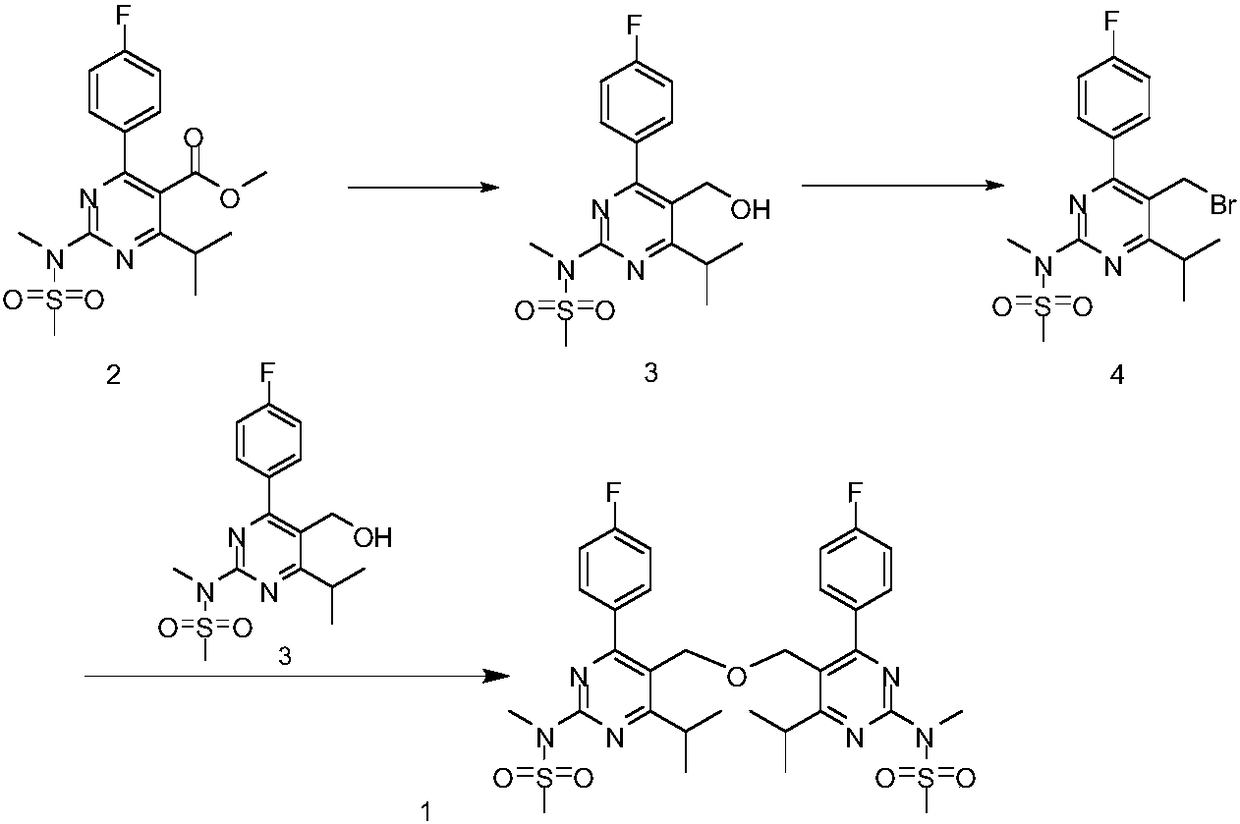
[0015] Preparation of 4-(4-fluorophenyl)-6-isopropyl-2-[(N-methyl-N-methanesulfonyl)amino]pyrimidine-5-methanol (3)
[0016]
[0017] Add 4-(4-fluorophenyl)-6-isopropyl-2-[(N-methyl-N-methanesulfonyl)amino]pyrimidine-5-carboxylic acid methyl ester (2) to a 1000ml three-necked flask (95.4g, 0.25mol), methanol 477ml, start stirring, add sodium borohydride (23.7g, 0.625mol) in batches at room temperature, after the addition, slowly heat up to reflux and stir the reaction, point TLC to detect, it is found that the reaction 4h raw materials disappeared. After the reaction is over, cool down to 0~10℃, add 20% dilute hydrochloric acid dropwise to adjust the system pH=4~5, filter by suction, and concentrate by the hydraulic pressure until almost no distillate flows out. Add 500ml dichloromethane and 300ml purified water to the residue and stir. Separate the liquids, wash the organic phase with 300 ml purified water once, collect the organic phase, concentrate under reduced pressure to dr...
Embodiment 2
[0020] Preparation of (5-(bromomethyl)-4-(4-fluorophenyl)-6-isopropyl-2-[methyl(methylsulfonyl)amino]pyrimidine (4)
[0021]
[0022] Add 4-(4-fluorophenyl)-6-isopropyl-2-[(N-methyl-N-methanesulfonyl)amino]pyrimidine-5-methanol (3) (70g, 0.2mol) and 420ml of dichloromethane, cooled to 0-10°C in an ice bath with stirring, dropwise add phosphorus tribromide (59.0g, 0.22mol) to the reaction system, stirred for 1h, and TLC detected the completion of the reaction. Add 250ml of water and stir to separate the liquids. Wash the organic layer with 200ml of 8% sodium bicarbonate solution and 200ml of purified water respectively. Collect the organic phase and concentrate to dryness under reduced pressure. Add 200ml of petroleum ether to the residue to be slurried at room temperature for 2h, and filter. The filter cake was dried to obtain 75.4 g of the compound (4) with a yield of 90.6%. HPLC detected 98.9%.
[0023] 1 HNMR (600MHz, DMSO-d6, δppm): 7.82 (m, 2H), 7.25 (m, 2H), 4.51 (s, 2H), 3...
Embodiment 3
[0025] N,N'-(5,5'-(oxybis(methylene))bis(4-(4-fluorophenyl)-6-isopropylpyrimidine-5,2-diyl))bis(N -Methanesulfonamide) (1) Preparation
[0026]
[0027] Add (5-(bromomethyl)-4-(4-fluorophenyl)-6-isopropyl-2-[methyl(methylsulfonyl)amino]pyrimidine (4) (70g, 0.17mol), 4-(4-fluorophenyl)-6-isopropyl-2-[(N-methyl-N-methanesulfonyl)amino]pyrimidine-5-methanol (3) (63.6g, 0.18 mol), potassium carbonate (47.0g, 0.34mol) and toluene 560ml, stir, stir and heat to reflux reaction for 12h, TLC test the end of the reaction, use 20% dilute hydrochloric acid to adjust the system pH = 6-7, suction filter, filter cake add 420ml methanol Recrystallization, filtration and drying to obtain 95.4 g of the target product (1) with a yield of 82.4%, 99.2% by HPLC.
[0028] 1 HNMR (600MHz, DMSO-d6, δppm): 7.80 (m, 4H), 7.24 (m, 4H), 4.50 (s, 4H), 3.56 (s, 6H), 3.46 (s, 6H), 1.42 (d, 12H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com