Method for performing chemical separation, analysis and test on gold in antimony electrolyte
A chemical separation, analysis and testing technology, applied in the field of chemical analysis, can solve the problems affecting the quality of cathode antimony products, complex components of antimony electrolyte, high salt concentration, etc., and achieve the effect of reliable analysis and testing method, strong timeliness and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
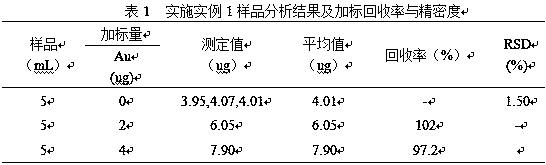
Embodiment 1
[0018] (1) Accurately pipette 5.0mL antimony electrolyte sample into a 400mL beaker, flush with water to keep the volume of the solution at 20mL, add 4mol / L dilute hydrochloric acid dropwise, and adjust the solution H + The concentration is 0.5mol / L, and then heated to 120°C to boil the solution for 2 minutes to make the precipitate flocculate and reunite, then stand and filter;
[0019] (2) Put the precipitate filtered in (1) together with the filter paper into the original beaker, add 20mL sulfuric acid with a concentration of 18mol / L and heat it to 300-380°C for 10-20min. After the sulfuric acid solution becomes transparent, Remove and cool to 20°C, add water to dilute to 10mL, add 20mL of hydrochloric acid with a concentration of 12mol / L, heat to 100°C and boil the solution for 5 minutes to dissolve all the salts, remove and cool, and let stand and filter;
[0020] (3) Put the precipitate filtered in (2) together with the filter paper into a beaker, add 10mL of nitric-sulf...
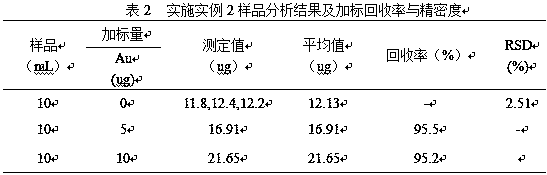
Embodiment 2
[0023] (1) Accurately pipette 10.0mL antimony electrolyte sample into a 400mL beaker, add water to keep the volume of the solution at 30mL, then add 4mol / L dilute hydrochloric acid dropwise to adjust the solution H + The concentration is 1mol / L, and then heated to 100°C to boil the solution for 5 minutes to make the precipitate flocculate and reunite, then stand and filter;
[0024] (2) Put the precipitate filtered in (1) together with the filter paper into a beaker, add 20mL of sulfuric acid with a concentration of 18mol / L and heat it to 370°C for 15min. After the sulfuric acid solution is transparent, take it out and cool it to 23°C, add water to dilute to a volume of 20mL, add 20mL of hydrochloric acid with a concentration of 12mol / L, and heat to 130°C to boil the solution for 8 minutes to dissolve all the salts, then take it out to cool, and let it stand and filter;
[0025] (3) Put the precipitate filtered in (2) together with the filter paper into a beaker, add 15mL of n...
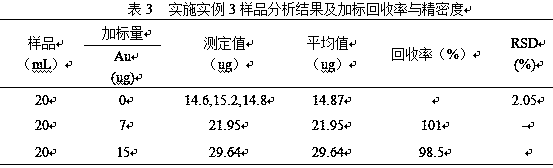
Embodiment 3
[0028] (1) Accurately pipette 20.0mL antimony electrolyte sample into a 400mL beaker, add water to keep the volume of the solution at 50mL, then add 4mol / L dilute hydrochloric acid dropwise to adjust the solution H + The concentration is 1mol / L, and then heated to 115 ° C to boil the solution for 3 minutes to make the precipitate flocculate and reunite, then stand and filter;
[0029] (2) Put the precipitate filtered in (1) together with the filter paper into a beaker, add 20mL of sulfuric acid with a concentration of 18mol / L and heat it to 380°C for 20min. After the sulfuric acid solution is transparent, take it out and cool it to 25°C, add water to dilute to a volume of 20mL, add 20mL of hydrochloric acid with a concentration of 12mol / L, and heat to 150°C and boil the solution for 10min to dissolve all the salts, then take it out to cool, and let it stand and filter;
[0030] (3) Put the precipitate filtered in (2) together with the filter paper into a beaker, add 20mL of ni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com