Preparation and use of 2-substituted-(S)-(3-mercapto-2-methylpropanoyl)-glycine derivatives
A technology of methacryl and derivatives, which is applied in the field of new pan-MBL inhibitors, can solve problems such as spreading, and achieve the effects of good reversal, low energy consumption and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1, Synthesis of (R)-2-(4-fluorophenyl)-2-((S)-3-thiomethyl-2-methylpropionamido)acetic acid (1)
[0029]
[0030] As shown in the synthesis diagram above, first, add D-p-fluorophenylglycine (200mg, 1.18mmol) into a 50ml double-necked bottle, add 15ml of methanol to dissolve, and slowly add SOCl dropwise at 0°C 2 (129μL, 1.77mmol), after dropping, after 10min, transfer to 75°C and heat to reflux for 2h. TLC monitored the completion of the reaction, and directly spin-dried to obtain 249mg of gray solid, with a yield of 96.4%.
[0031] Then, the product obtained in the first step (200mg, 1.09mmol) was dissolved in 4ml DMF, and then HOBT (301mg, 2.18mmol), EDCI (417mg2.18mmol), DIEA (569μL, 3.27mmol), (S)-2 -Methylacetylated mercaptopropionic acid (177mg, 1.09mmol) was stirred at room temperature for 12h, and the reaction was completed. Add saturated NH 4 Cl solution, EA extracted three times, the combined organic layer was washed once with saturated brine, drie...
Embodiment 2
[0034] Example 2, Synthesis of (S)-2-(4-fluorophenyl)-2-((S)-3-thiomethyl-2-methylpropionamido)acetic acid (2)
[0035]
[0036] The synthesis method was similar to Example 1, and the target compound 2 was obtained, and the total yield of the three steps was 46.0%. 1 H NMR (400 MHz, DMSO- d 6 ) δ 12.83 (s, 1H), 8.66 (d, J = 7.4 Hz, 1H), 7.44 (d, J= 5.2 Hz, 2H), 7.21 (t, J = 8.4 Hz, 2H), 5.37 (d, J = 7.1 Hz, 1H), 2.63 (d, J = 7.3 Hz, 2H),2.47-2.37 (m, 1H), 2.08 (t, J = 7.4 Hz, 1H), 1.08 (d, J = 5.3 Hz, 3H) ppm; 13 CNMR (150 MHz, DMSO- d 6 ) δ 174.31, 172.23, 133.79, 133.60, 130.20, 130.14, 115.77, 115.63, 55.91, 43.22, 27.69, 17.61 ppm. LC-MS: m / z 270.1 [M - H] - .
Embodiment 3
[0037] Example 3, Synthesis of (R)-2-(4-hydroxyphenyl)-2-((S)-3-thiomethyl-2-methylpropionamido)acetic acid (3)
[0038]
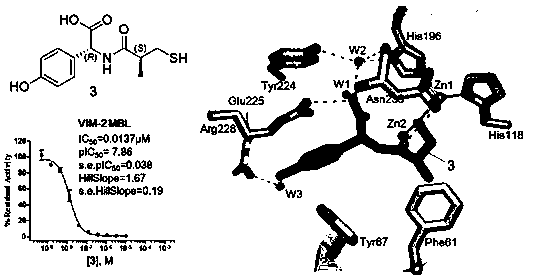
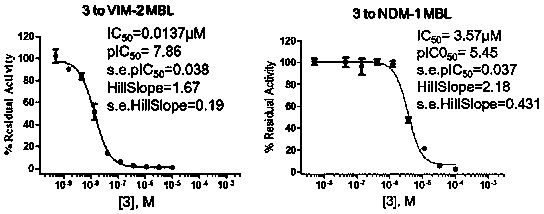
[0039] The synthesis method was similar to Example 1, and the target compound 3 was obtained, and the total yield of the three steps was 49.2%. 1 H NMR (400 MHz, CDCl 3 ) δ 12.57 (s, 1H), 9.50 (s, 1H), 8.50 (d, J = 7.0 Hz, 1H), 7.18 (d, J =8.2 Hz, 2H), 6.76 (d, J = 8.0 Hz, 2H), 5.17 (d, J = 7.1 Hz, 1H), 2.75-2.57(m, 2H), 2.41-2.38 (m, 1H), 2.19 (t, J = 7.5 Hz, 1H), 1.02 (d, J = 6.4 Hz,3H) ppm; 13 C NMR (100 MHz, CDCl 3 ) δ 178.98, 177.57, 162.32, 134.04, 133.84,132.35, 120.36,120.31, 60.97, 47.74, 32.80, 22.11 ppm. LC-MS: m / z 268.1 [M-H] - .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com