Preparation process of solvent dye
A preparation process and a technology of solvent dyes, which are applied in the preparation process of 2-indan-1,3-dione and the field of solvent dyes, and can solve the problem of difficult control of the amount of bromine used, high energy consumption and high consumption of recycled solvents. It can improve the uniformity of dyeing, reduce the difficulty of processing and use economically.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
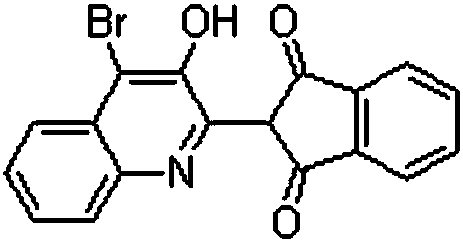
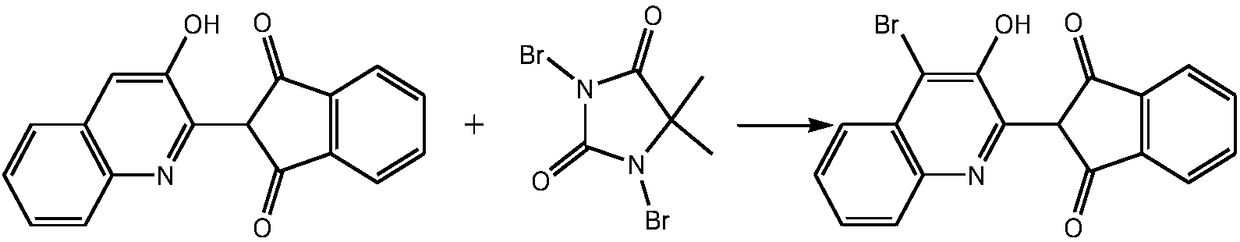
[0017] In a 500mL four-neck flask equipped with an electric stirrer, add 3.18g Pingpingo, 79.5g 2-(3-hydroxy-2-quinolyl)-1H-indene-1,3(2H)-dione and 126mL of dichloromethane, stirred and raised to 40°C, 159g of 1,3-dibromo-5,5-dimethylhydantoin was added in 3 batches, and the temperature was raised to reflux for 5h. After the reaction, filter, wash, and dry to obtain 96.57 g of the target product. The yield is 95.4%, the purity is 98.0%, and the tinting strength is 100.15%.
Embodiment 2
[0019] In a 500mL four-neck flask equipped with an electric stirrer, add 79.5g 2-(3-hydroxy-2-quinolyl)-1H-indene-1,3(2H)-dione, 4g Pingjiao and 150mL Dichloromethane, stirred and raised to 40°C, 170.93g of 1,3-dibromo-5,5-dimethylhydantoin was added in 4 batches, and the temperature was raised to reflux for 6 hours. After the reaction was finished, filter, wash, and dry to obtain 97.48 g of the target product. The yield is 96.3%, the purity is 98.24%, and the tinting strength is 100.73%.
Embodiment 3
[0021] In a 500mL four-neck flask equipped with an electric stirrer, add 7.95g Pingpingo O, 180mL dichloromethane, 79.5g 2-(3-hydroxy-2-quinolinyl)-1H-indene-1,3(2H )-diketone, stirred, heated to reflux, added 178.88g of 1,3-dibromo-5,5-dimethylhydantoin in 5 batches, stirred and heated to 40°C, then heated to reflux for 8 hours. After the reaction, filter, wash, and dry to obtain 98.7 g of the target product. The yield is 97.5%, the purity is 98.70%, and the tinting strength is 100.89%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| tinctorial strength | aaaaa | aaaaa |
| tinctorial strength | aaaaa | aaaaa |
| tinctorial strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com