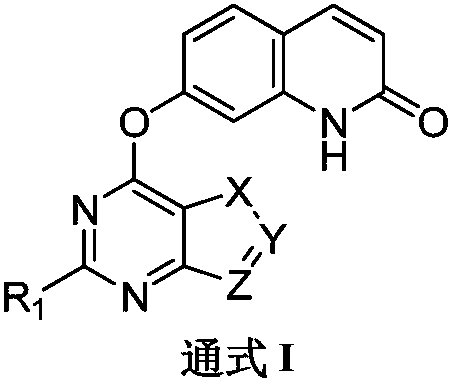
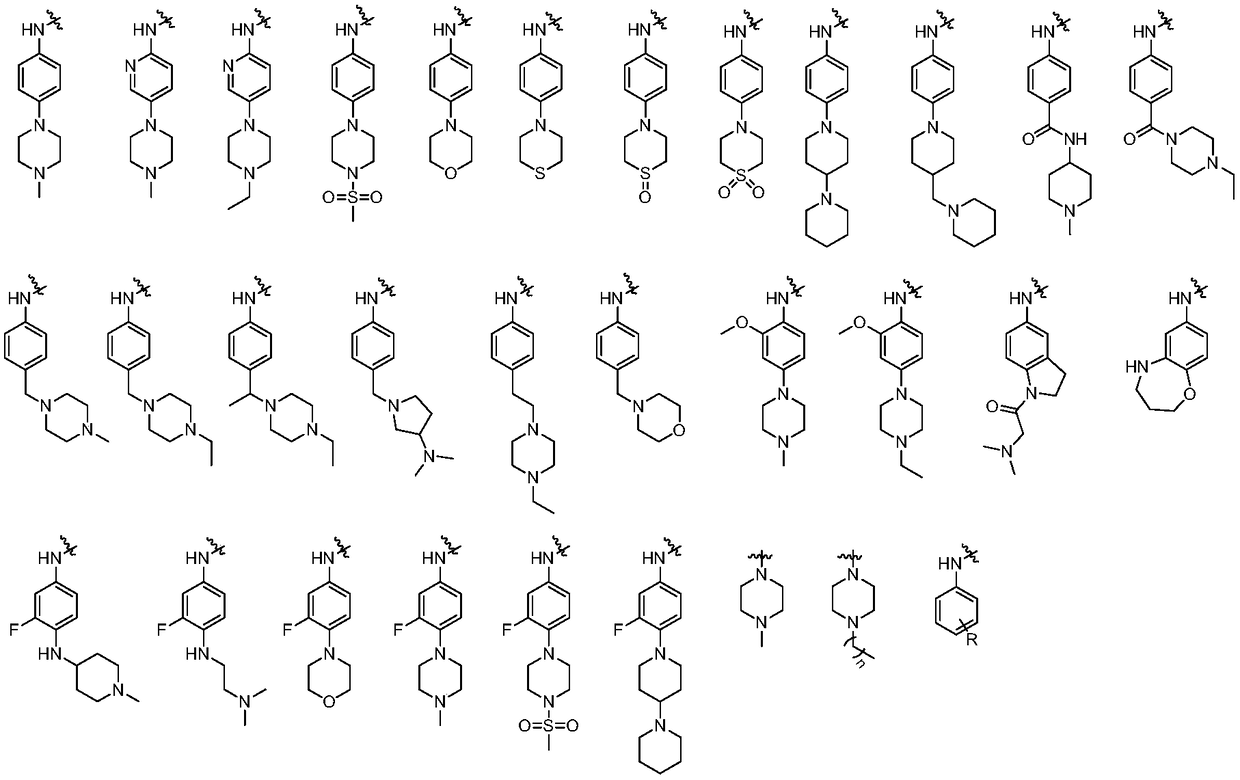
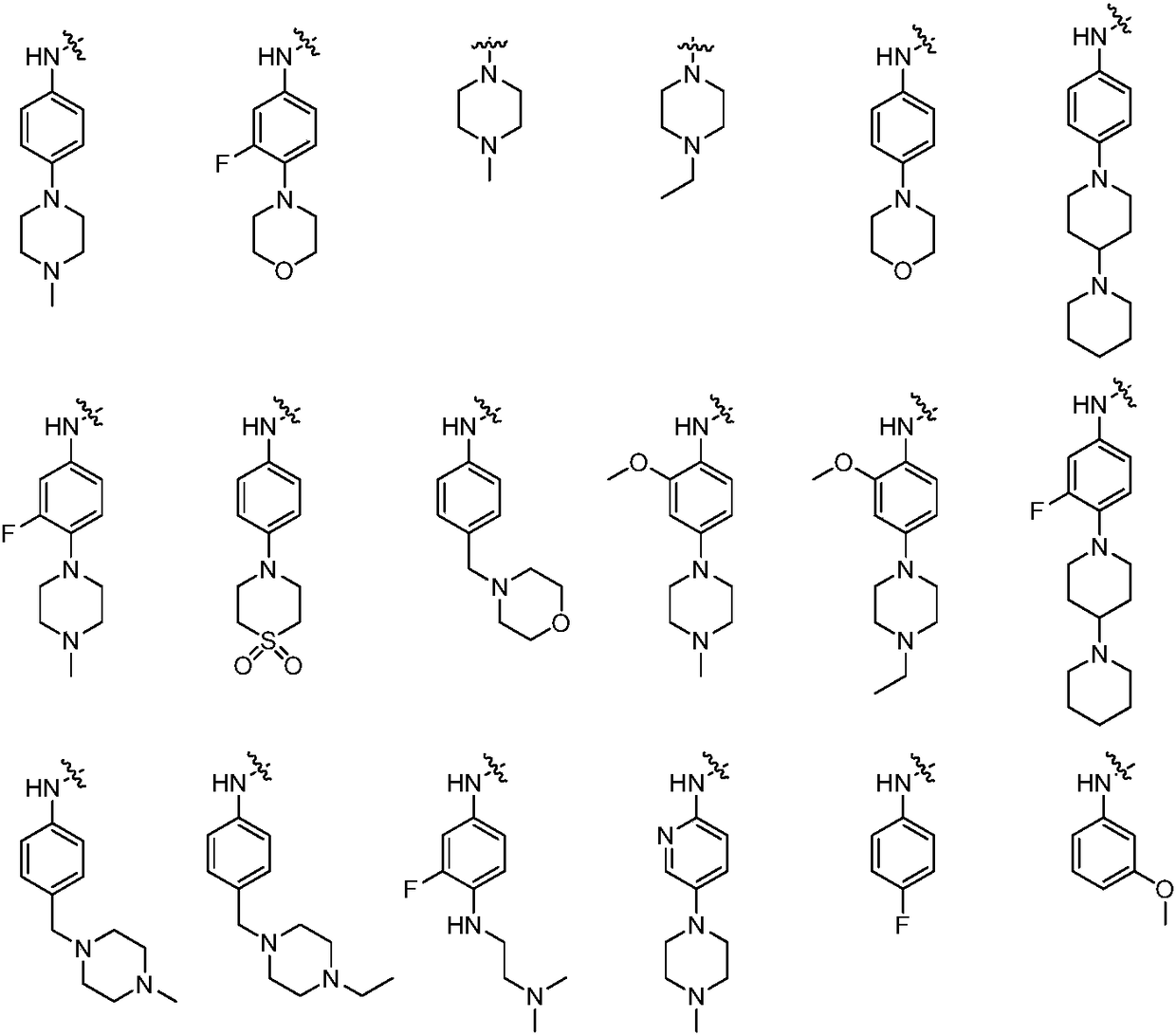
Quinolinone-containing pyrimido-five-membered heterocyclic compounds and a preparation method and application thereof
A technology for five-membered heterocycles and compounds, which is applied in the field of novel pyrimido five-membered heterocycles containing quinolinones, which can solve the problems of reduced drug efficacy and achieve the effect of simple and feasible synthetic methods and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: the preparation of compound 3
[0031]
[0032] To a mixed solution of acetone (8mL) and water (16mL), add m-methoxyaniline (1g, 8.12mmoL), potassium carbonate (1.68g, 12.18mmoL), cinnamoyl chloride (1.62g, 9.74mmoL) successively, ice The bath was stirred for 30 min, and the reaction was monitored by TLC. After the reaction, the reaction system was poured into ice water, extracted three times with ethyl acetate, the organic phases were combined, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain compound 3 with a yield of 98%. White solid,Mp 114-115℃. 1 H NMR (400MHz, DMSO-d 6 )δ10.19(s,1H),7.61(dd,J=13.0,12.1Hz,3H),7.49–7.38(m,4H),7.22(dd,J=8.8,3.9Hz,2H),6.83(d ,J=15.7Hz,1H),6.69–6.62(m,1H),3.75(s,3H). 13 C NMR (100MHz, DMSO-d 6 )δ163.53, 159.52, 140.39, 140.20, 134.68, 129.76, 129.55, 128.99, 127.69, 122.23, 111.55, 108.76, 105.08, 54.95.
Embodiment 2
[0033] Embodiment 2: the preparation of compound 4
[0034]
[0035] Under ice bath, aluminum trichloride (3.16g, 23.69mmoL) was added in batches to the suspension of compound 3 (1g, 3.95mmoL) in chlorobenzene (30mL), and the reaction system was gradually heated to 120°C and refluxed for 3-5h , TLC monitors the reaction system. After the reaction, the reaction system was evaporated to dryness under reduced pressure, separated and purified by silica gel column chromatography to obtain compound 4 with a yield of 54%. Brownsolid,Mp>300℃. 1 H NMR (400MHz, DMSO-d 6 )δ11.50(s,1H),10.11(s,1H),7.73(d,J=9.4Hz,1H),7.44(d,J=8.5Hz,1H),6.72–6.56(m,2H), 6.21(d,J=9.4Hz,1H). 13 C NMR (100MHz, DMSO-d 6 )δ162.29, 160.06, 141.05, 140.45, 129.42, 116.07, 112.94, 112.46, 100.09.
Embodiment 3
[0036]Embodiment 3: the preparation of compound 5
[0037]
[0038] Compound 4 (0.7g, 4.34mmoL) and 2,4-dichlorothieno[3,2-d]pyrimidine (1.07g, 5.21mmoL) were added to the reaction flask, and then potassium carbonate (1.8g, 13.03mmoL ), water (10mL), acetonitrile (50mL), reflux at 70°C for 2-5h, and monitor the reaction system by TLC. After the reaction, a white solid was precipitated, cooled, suction filtered, the filter cake was washed several times with dichloromethane and water, and dried in a vacuum oven to obtain compound 5 with a yield of 78%. White solid,Mp>300℃. 1 H NMR (400MHz, DMSO-d 6 )δ8.57(d, J=5.4Hz, 1H), 7.90(d, J=9.5Hz, 1H), 7.75(d, J=8.6Hz, 1H), 7.65(d, J=5.4Hz, 1H) ,7.24(d,J=2.2Hz,1H),7.14(dd,J=8.5,2.3Hz,1H),6.50(d,J=9.5Hz,1H). 13 C NMR (100MHz, DMSO-d 6 )δ164.43, 163.92, 163.05, 154.81, 152.33, 141.50, 139.72, 139.15, 129.33, 123.51, 121.73, 117.73, 116.29, 115.28, 108.54.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com