Impurity separation and detection method for telmisartan
A technology of telmisartan and detection method, which is applied in the field of telmisartan impurity separation and detection, can solve problems such as difficult separation and inability to achieve complete separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0128] Step 1, solution preparation:
[0129] Take an appropriate amount of telmisartan raw material or preparation powder, add 100 μl of 1mol / L sodium hydroxide to aid dissolution, dilute with methanol to 0.5mg / ml, and make the test solution;
[0130] Dilute it again as needed to make a 5 μg / ml solution as its own reference solution;
[0131] Step 2, system suitability solution preparation:
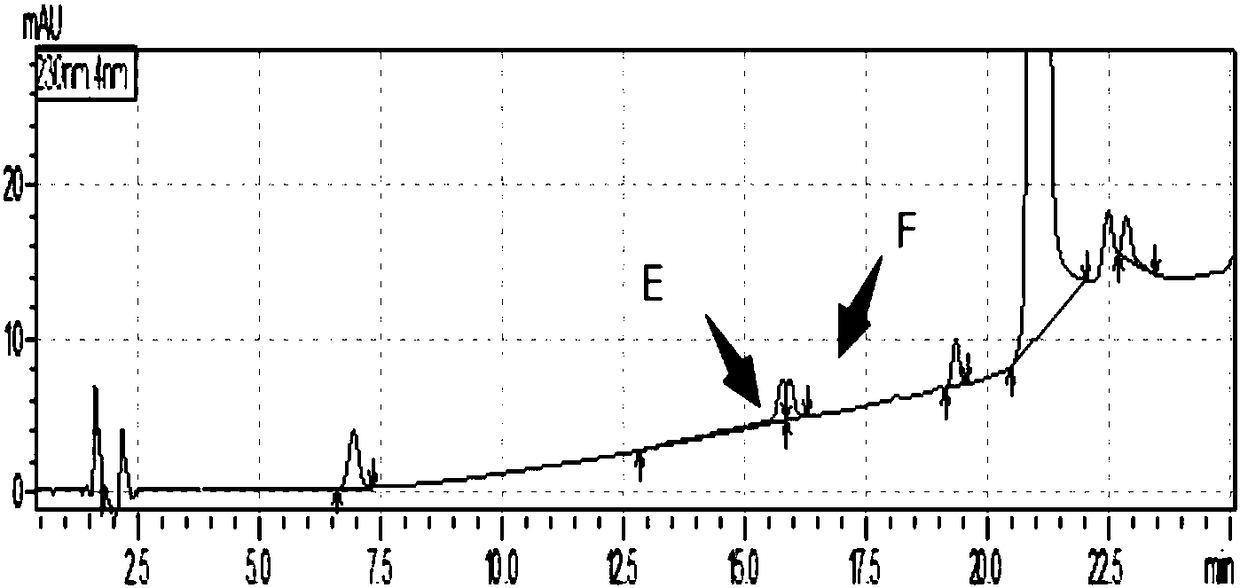
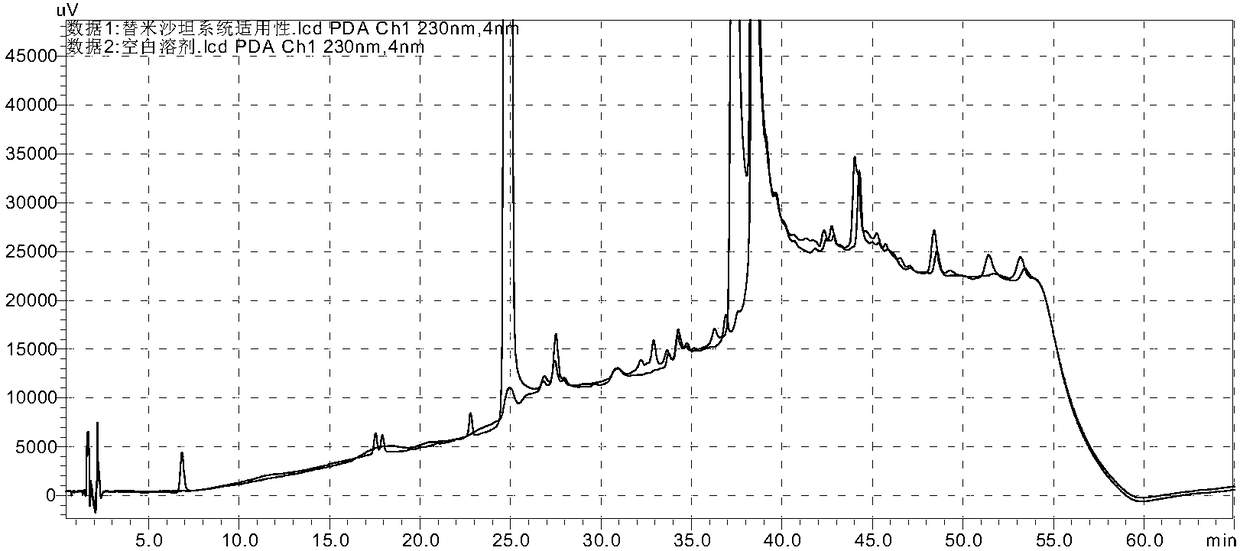
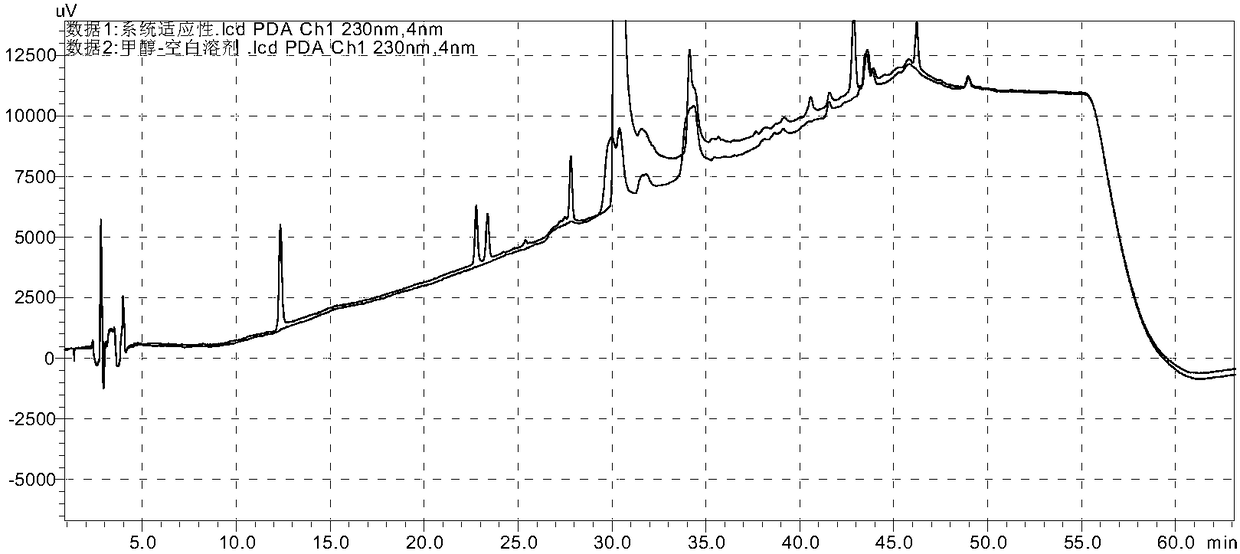
[0132] Take each appropriate amount of each impurity reference substance (A, B, C, E, F, G, H, I, intermediate ester), dissolve it with methanol and quantitatively dilute it to make a solution containing 1 mg per 1 ml, and store it as each impurity reference substance solution; measure each impurity reference substance (A, B, C, E, F, G, H, I, intermediate ester) stock solution 1ml and telmisartan raw material or preparation powder amount, add methanol quantitative dilution to make each 1ml of a mixed solution containing 2 μg of each impurity and 0.5 mg of telmisartan is used as a syst...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com