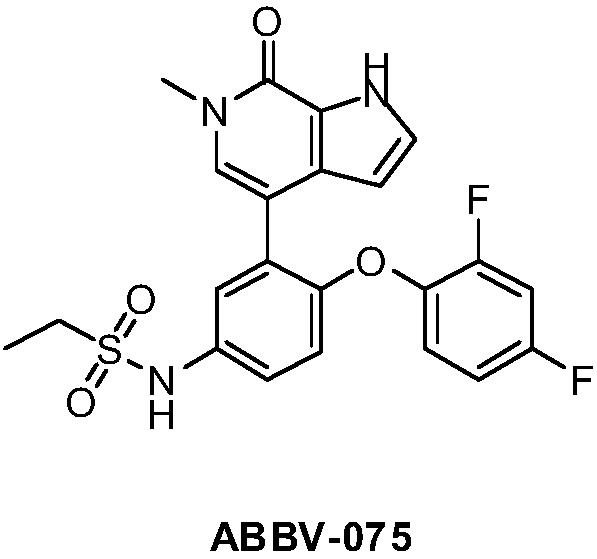
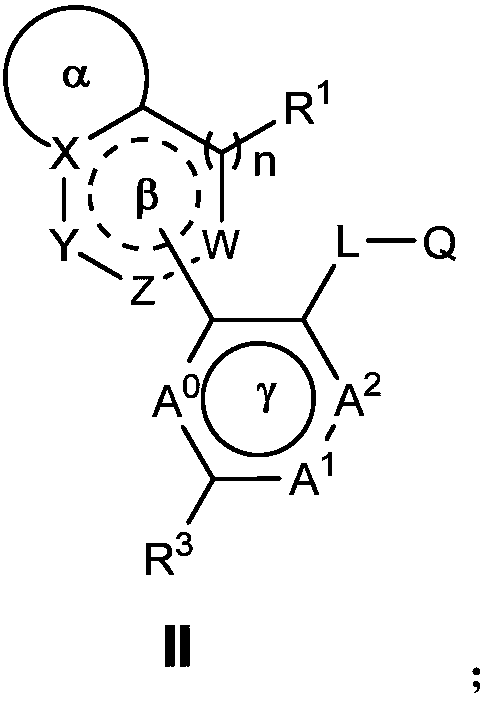
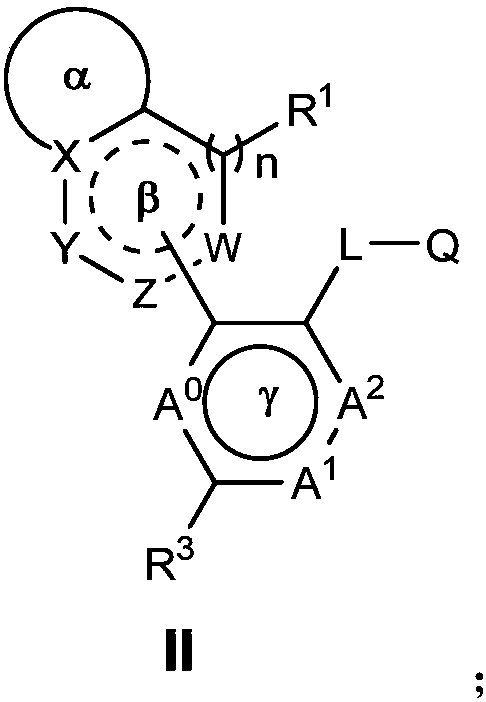
Nitrogen-containing heterocyclic ring compounds, and preparation method, pharmaceutical compositions and application thereof
A nitrogen-heterocycle and compound technology, applied in the field of nitrogen-containing heterocycle compounds, can solve problems such as weak binding between bromodomain and acetylated protein, and influence on druggability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0292] Example 1: N-(4-(2,4-Difluorophenoxy)-3-(1-oxoisoindolin-5-yl)phenyl)ethylsulfonamide (II-1)
[0293]
[0294] Step 1: 2-Bromo-1-(2,4-difluorophenoxy)-4-nitrobenzene
[0295]
[0296] Dissolve 2-bromo-1-fluoro-4-nitrobenzene (6.60g, 30.00mmol), 2,4-difluorophenol (3.90g, 30.00mmol) and cesium carbonate (19.50g, 60.00mmol) in dimethyl Sulfoxide (70 mL) and stirred at 110 °C for 2 h. The reaction solution was poured into water (100 mL), and extracted with ethyl acetate (60*3 mL). The organic phase was washed with water and brine, dried (anhydrous sodium sulfate), filtered and concentrated to obtain the crude title compound (9.90 g, 100%), which was directly used in the next reaction.
[0297] Step 2: 3-bromo-4-(2,4-difluorophenoxy)aniline
[0298]
[0299] Compound 1A (9.9g, 30.00mmol), iron powder (8.40g, 150.00mmol), and ammonium chloride (3.21g, 60.00mmol) were dissolved in a mixture of tetrahydrofuran / ethanol / water (45mL / 45mL / 15mL) , Stir at 100°C for 2h. The solid was fi...
Embodiment 2
[0305] Example 2: N-(4-(2,4-Difluorophenoxy)-3-(1-oxoisoindolin-4-yl)phenyl)ethylsulfonamide (II-2)
[0306]
[0307] Step 1: N-(4-(2,4-Difluorophenoxy)-3-(1-oxoisoindolin-4-yl)phenyl)ethylsulfonamide
[0308] Compound 1C (100mg, 0.26mmol), 4-(4,4,5,5-tetramethyl-1,3,2-dioxoborolan-2-yl)isoindolin-1-one (85mg, 0.33mmol), [1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium(II) (20mg, 0.03mmol) and potassium carbonate (88mg, 0.64mmol) dissolved in 1, In 4-dioxane (2mL), and stirred overnight at 110°C under nitrogen protection. The reaction solution was concentrated, and the residue was separated and purified by flash chromatography (petroleum ether / ethyl acetate = 2 / 1) using the residue to obtain the title compound (80 mg, 71%) as a white solid. MS(ESI): m / z=391.2[M+1] + . 1 H NMR(400MHz, DMSO-d 6 )δ9.81(s,1H),8.63(s,1H),7.67(s,1H),7.56(s,2H),7.30(t,J=19.9Hz,3H),7.02(d,J=24.7 Hz, 3H), 4.33 (s, 2H), 3.14 (s, 2H), 1.22 (s, 3H).
Embodiment 3
[0309] Example 3: N-(4-(2,4-Difluorophenoxy)-3-(3-methyl-2-oxo-1,2,3,4-tetrahydroquinazoline-6- (Yl)phenyl)ethylsulfonamide (II-3)
[0310]
[0311] Step 1: 4-(2,4-Difluorophenoxy)-3-(4,4,5,5-tetramethyl-1,3,2-dioxoborolan-2-yl)aniline
[0312]
[0313] Compound 1B (3.00g, 10.00mmol), pinacol diborate (5.18g, 20.00mmol), 1,1'-bis(diphenylphosphino)ferrocene)dichloropalladium(II) (732mg ,1.00mmol) and potassium acetate (3.00g, 30.00mmol) were dissolved in 1,4-dioxane (50mL), and the reaction solution was stirred at 100°C under nitrogen protection for 2h. The reaction solution was concentrated, and the residue was separated and purified by flash chromatography (ethyl acetate / petroleum ether = 1 / 9) to obtain the title compound (3.50 g, 90%) as a yellow oil. LCMS(ESI)[M+H] + =348.2.
[0314] Step 2: 6-Bromo-3-methyl-3,4-dihydroquinazolin-2(1H)-one
[0315]
[0316] To a solution of 3-methyl-3,4-dihydroquinazolin-2(1H)-one (1.0g, 61.7mmol) in dichloromethane (25mL) was slowly added N-br...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com