Zinc oxide-based carbon dioxide reduction electrocatalyst and preparation method thereof
An electrocatalyst, carbon dioxide technology, applied in the direction of metal/metal oxide/metal hydroxide catalyst, carbon monoxide, physical/chemical process catalyst, etc., can solve the complicated synthesis process, high starting potential, and harsh conditions for carbon dioxide chemical conversion reaction and other problems, to achieve the effect of simple preparation method, low onset potential and excellent catalytic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Add 0.5 mol Zn(NO 3 ) 2 ·6H 2 O was dissolved in deionized water to obtain 10 mL of Zn(NO 3 ) 2 solution, 4 mol KOH was dissolved in deionized water to obtain 10 mL of KOH solution with a concentration of 4 mol / L. At a stirring rate of 600 rpm, the Zn(NO 3 ) 2 The solution was added dropwise to the KOH solution until the Zn(NO 3 ) 2 The entire solution is transferred;
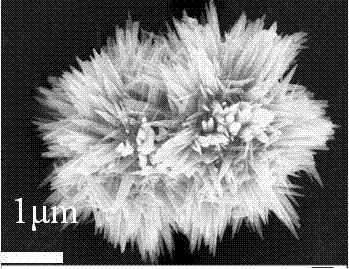
[0029] (2) Place the above suspension in an airtight container at 50 o C was reacted for 12 h, and the precipitate obtained by the reaction was washed with absolute ethanol and deionized water successively and centrifuged, and the washing was stopped when the pH value of the centrifuged solution reached 7.5. o C was dried in vacuum for 9 h to obtain dandelion-like ZnO nanomaterials.
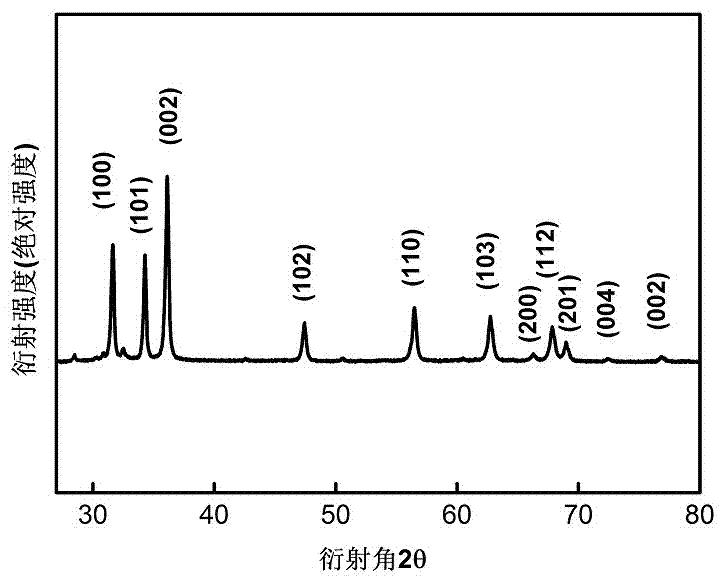
[0030] The zinc oxide nanomaterials provided in the above embodiments of the present invention were characterized by XRD and SEM. figure 1 It is the XRD characterization result, the peak position of each crys...
Embodiment 2
[0034] (1) Add 0.5 mol Zn(CH 3 COO) 2 Dissolved in deionized water to obtain 10 mL of Zn(CH 3 COO) 2 solution, 4 mol KOH was dissolved in deionized water to obtain 10 mL of KOH solution with a concentration of 4 mol / L. At a stirring rate of 600rpm, the Zn(CH 3 COO) 2 The solution is directly poured into the KOH solution;
[0035] (2) Put the above suspension in an airtight container at 40 o C was reacted for 10 h, and the precipitate obtained by the reaction was washed with absolute ethanol and deionized water successively and centrifuged, and the washing was stopped when the pH value of the centrifuged liquid reached 7.5. o C was dried in vacuum for 9 hours to obtain flaky zinc oxide nanomaterials.
Embodiment 3
[0037] (1) Add 0.5 mol ZnCl 2 Dissolve in deionized water to obtain 10 mL of ZnCl with a concentration of 0.5 mol / L 2 solution, 4 mol KOH was dissolved in deionized water to obtain 10 mL of KOH solution with a concentration of 4 mol / L. At a stirring rate of 600 rpm, the ZnCl 2 The solution was added dropwise to the KOH solution until the ZnCl 2 The entire solution is transferred;
[0038] (2) Put the above suspension in an airtight container at 60 o C was reacted for 15 h, and the precipitate obtained by the reaction was washed successively with absolute ethanol and deionized water and centrifuged, and the washing was stopped when the pH value of the centrifuged solution reached 7.5. o C was dried in vacuum for 9 h to obtain rod-shaped zinc oxide nanomaterials.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| electric potential / voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com