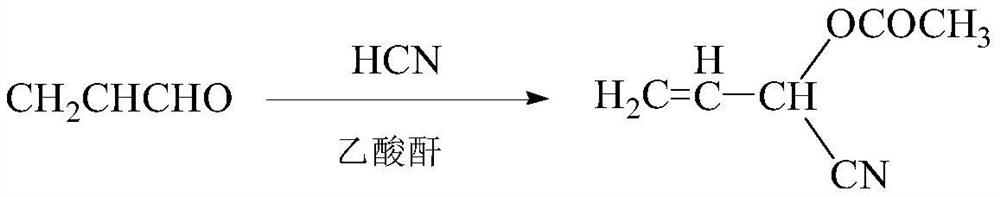
A method for synthesizing 2-acetoxy-3-butenenitrile
A technology of acetoxy and acrylonitrile, applied in the field of fine chemistry, can solve problems such as unfavorable reactions, processing requirements and high costs, and achieve the effects of reducing costs, improving efficiency, and environmental protection costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1 mol of hydrocyanic acid (27g) and 3 mol of methanol (96g) were mixed, and after mixing, the temperature was lowered to -10°C for later use. 1 mol (56 g) of acrolein and 1.01 mol (103.2 g) of acetic anhydride were mixed in a flask equipped with magnetic stirring, and the temperature was lowered to -15°C. Add the mixed solution of acrolein and acetic anhydride to the methanol solution of hydrocyanic acid dropwise for 2 hours. During the dropwise addition, keep the temperature of the flask at -10~-12°C. Finally, a rectification column with 3 trays is used, the substrate is heated, the top reflux ratio is 1:4, and methanol is obtained at 67°C, and acetic acid is obtained at 118°C. After methanol and acetic acid are extracted, the temperature at the bottom of the tower will increase , continue heating, and extract 125.4 g of 2-acetoxy-3-butenenitrile product at 170° C., the purity is 98.5%, and the yield reaches 98.8%.
Embodiment 2
[0024] 1.05 mol of hydrocyanic acid (28.35 g) and 3.15 mol of methanol (100.8 g) were mixed, and after mixing, the temperature was lowered to -10°C for later use. 1 mol (56 g) of acrolein and 1.2 mol (122.4 g) of acetic anhydride were mixed in a flask equipped with magnetic stirring, and the temperature was lowered to -15°C. Add the mixed solution of acrolein and acetic anhydride to the methanol solution of hydrocyanic acid dropwise for 2 hours. During the dropwise addition, keep the temperature of the flask at -10~-12°C. Finally, referring to the rectification conditions in Example 1, 125.5 g of 2-acetoxy-3-butenenitrile product was obtained with a purity of 98.8% and a yield of 99.2%.
Embodiment 3
[0026] 1 mol of hydrocyanic acid (27g) and 3 mol of methanol (96g) were mixed, and after mixing, the temperature was lowered to -10°C for later use. 1 mol (56 g) of acrolein and 1.01 mol (103.2 g) of acetic anhydride were mixed in a flask equipped with magnetic stirring, and the temperature was lowered to -5°C. Add the mixed solution of acrolein and acetic anhydride to the methanol solution of hydrocyanic acid dropwise for 2 hours. During the dropwise addition, keep the temperature of the flask at -2~0°C. After the dropwise addition, continue the heat preservation reaction for 1 hour. , with reference to the rectifying conditions of Example 1, 116.2 g of 2-acetoxy-3-butenenitrile product was obtained, the purity was 92.5%, and the yield reached 86%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com