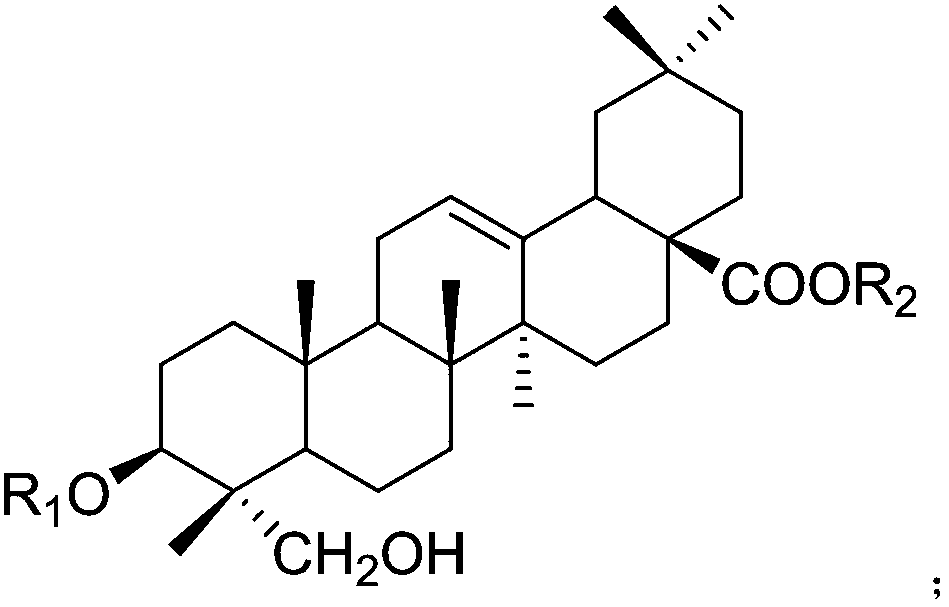
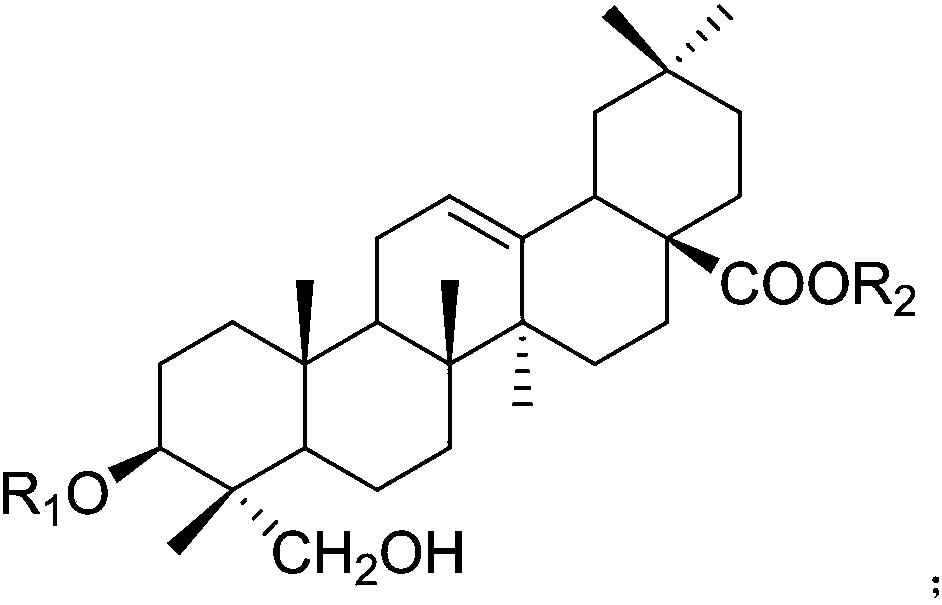
Application of hederagenin and glucoside thereof to preparation of medicine for treating osteoporosis
A technology for hederagenin and osteoporosis, which is applied in the field of medicine and can solve problems such as no reports of hederagenin and its glycoside osteoporosis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: The pharmacological action of helexin and its glycosides
[0044] 1. Experimental method
[0045] After 3-month-old nulliparous female SD rats were fed for 3 days, they were randomly divided into two groups, sham operation group and ovariectomized operation group. In the sham group, only a small amount of fat tissue around the ovaries was removed. Ovariectomy group underwent ovariectomy, and 400,000 IU / kg of penicillin was injected 3 days after operation to prevent infection.
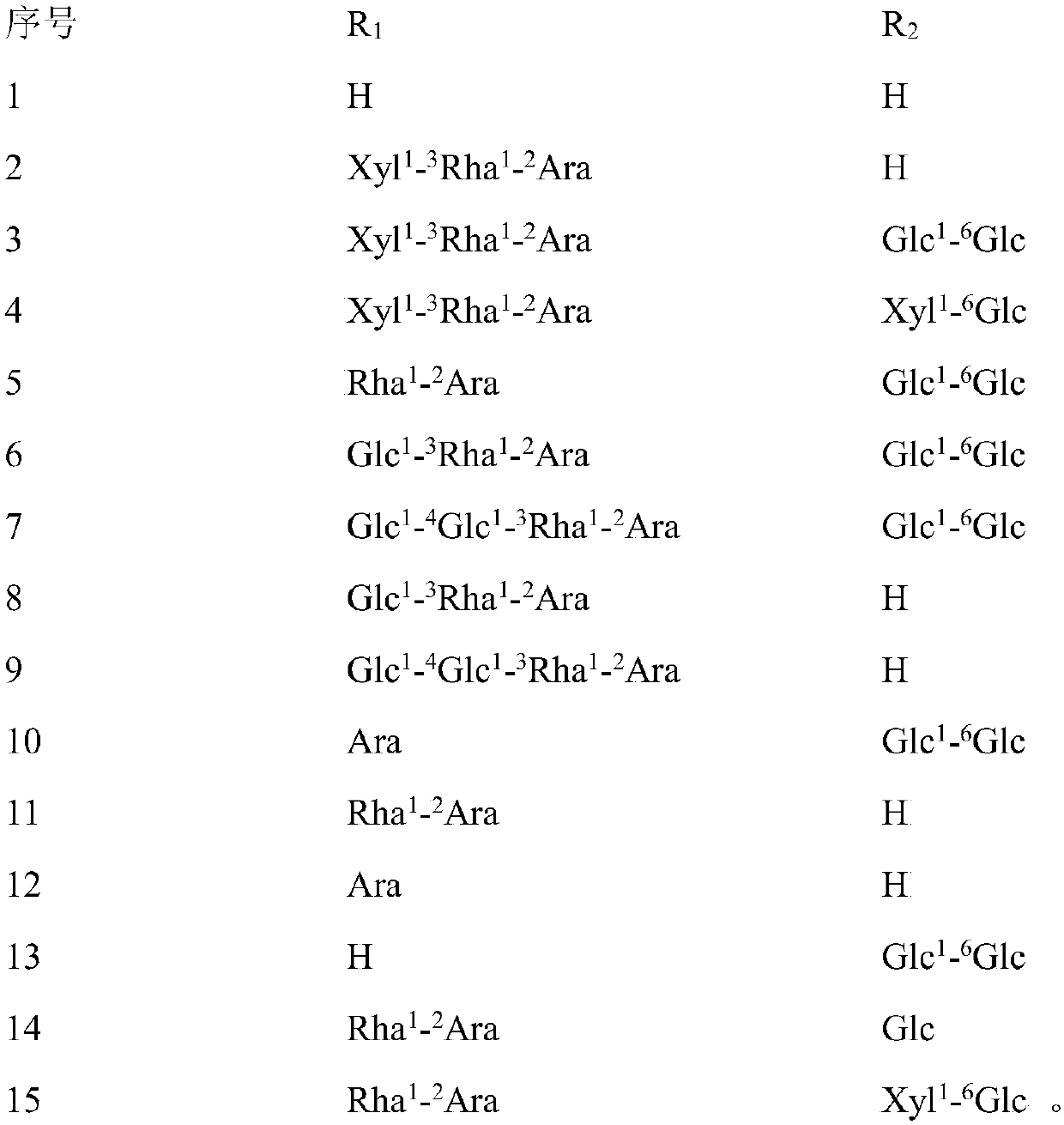
[0046]After the operation was completed and the rats woke up, the ovariectomized operation group was randomly divided into 16 groups: the model group and the helexin or saponin administration group with serial numbers 1-15, 10 rats in each group, and fed in separate cages. Feed was added and water was changed every day, and the survival status was observed. There was no statistical difference in the weight of rats in each group before administration. The rats basically recovered ...
Embodiment 2
[0059] Embodiment 2: Preparation and pharmacological action of an extract containing helexin or its glycosides
[0060] Part 1: Preparation of Extract
[0061] Preparation method one 5kg of dry Lonicera chinense medicinal material, reflux extraction with 70% ethanol for 3 times, 2h each time, filter, combine the filtrate, reclaim the solvent under reduced pressure to obtain extractum (get partly dried to obtain total extract 1), add appropriate amount of water to suspend , filtered, loaded on D101 macroporous resin, and started gradient elution from 10%, 30%, 50%, 70%, 90% ethanol, and monitored whether the eluate contained DecaisosideE, yellow Honeysuckle saponin B or Dipsacus saponin B, any of the three appear in the eluent and start to collect until all three are eluted from the resin, stop collecting, and concentrate and dry this part of the eluent to obtain the extract of Lonicera tomentosa 1.
[0062] Preparation method two 5kg of dry Lonicera chinensis medicinal ma...
Embodiment 3
[0085] Embodiment 3: pharmaceutical preparation
[0086] 1. Tablet: 5g of helexin or saponin or Lonicera tomentosa extract of serial number 1-15, 50g of starch, 3g of magnesium stearate. Preparation process: take the hedera saponin or saponin or the extract of Lonicera tomentosa with the serial number 1-15, add starch and magnesium stearate, mix evenly, make granules, dry, and compress into tablets.
[0087] 2. Capsules: 5g of hedera saponin or saponin or Lonicera tomentosa extract with serial number 1-15, 50g of starch, 3g of magnesium stearate. Preparation process: take the hedera saponin or saponin or Lonicera tomentosa extract of serial number 1-15, add starch and magnesium stearate, mix evenly, make granules, dry, and pack into capsules.
[0088] 3. Injection: 1g of hedera saponin or saponin or Lonicera tomentosa extract with serial number 1-15, appropriate amount of sodium chloride for injection. Preparation process: take the hedera saponin or saponin or Lonicera chrys...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com