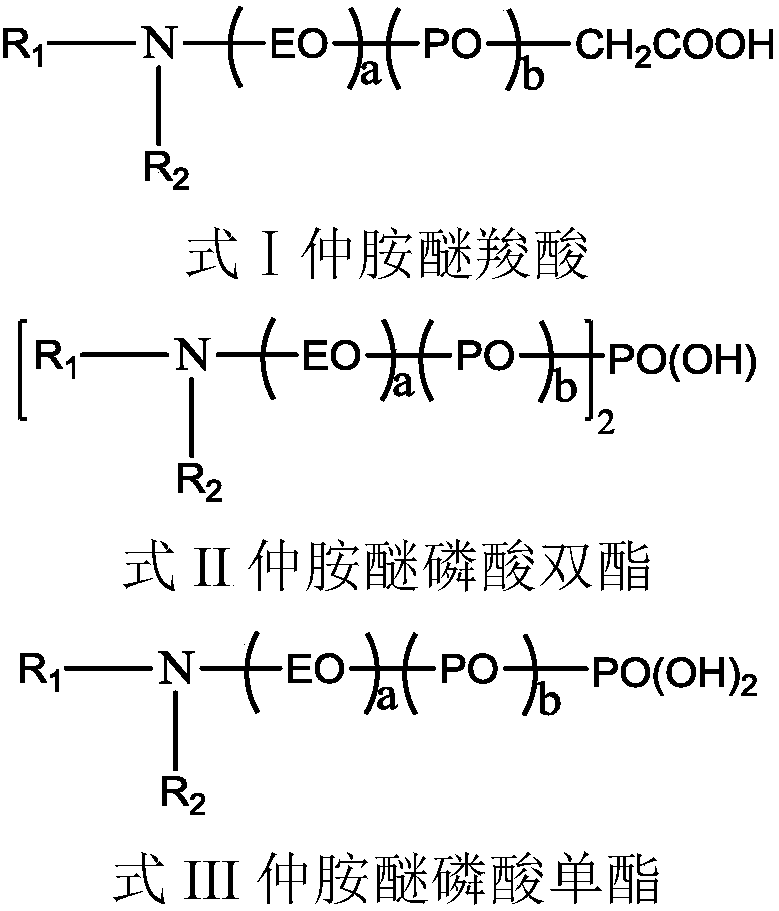
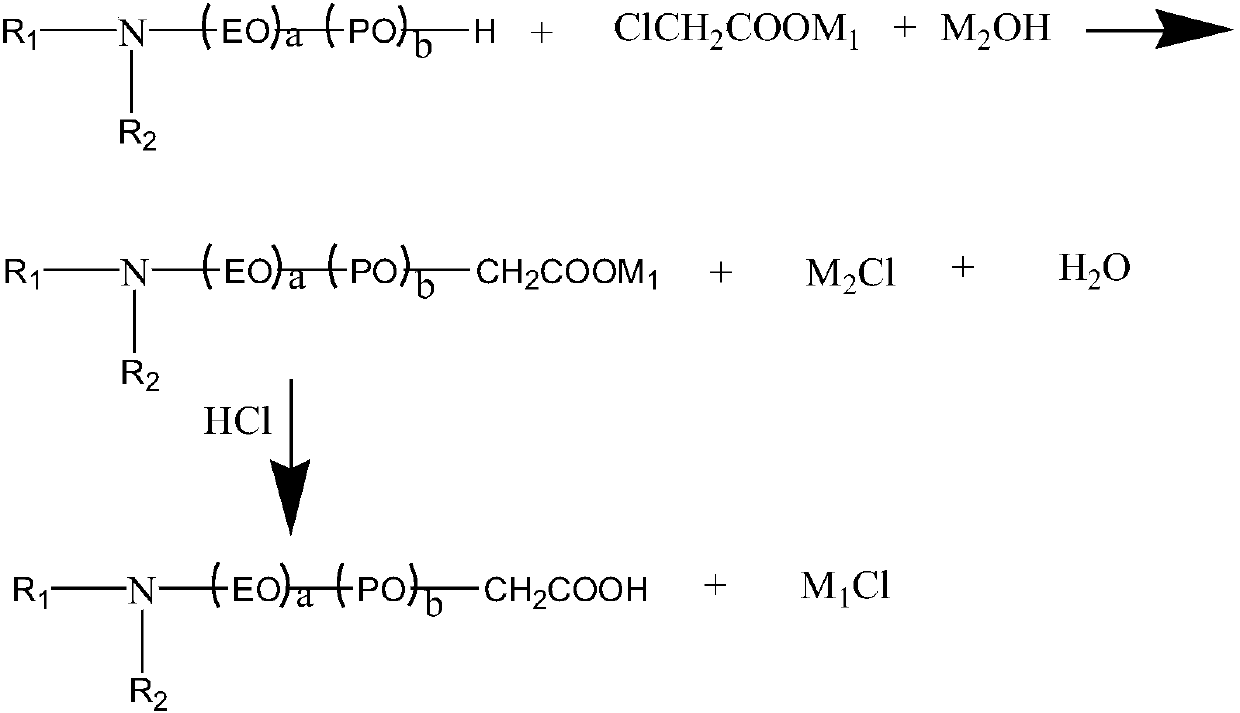
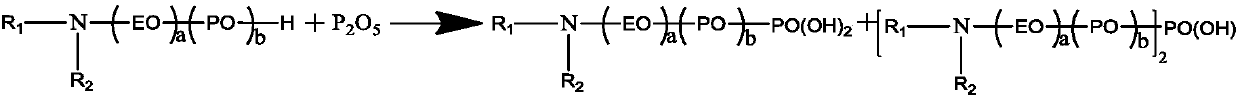Alkylamine ether derived surfactant and preparation method thereof
A technology of surfactants and alkylamine ethers, which is applied in the field of alkylamine ether derivative surfactants and their preparation, can solve the problems of poor compatibility of anionic surfactants, and achieve curb side reactions, easy operation, The effect of simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0034] The preparation of didodecanyl secondary amine ether (5EO) carboxylic acid shown in formula V
[0035]
[0036] A kind of preparation method of didodecanyl secondary amine ether (5EO) carboxylic acid, comprises the following steps: the first step: carboxylation reaction, earlier raw material 1.3mol didodecanyl secondary amine ether (5EO) and 1mol chlorine Sodium acetate was placed in the experimental device equipped with a stirrer, thermometer, water separator and condenser tube. The whole experimental device was inerted for 3 times, and finally nitrogen protection was introduced, the condenser was opened, and the temperature was raised to 40-90 ° C. Sodium hydroxide is evenly divided into 5-10 parts, and added in batches and evenly within 8-20 hours of reaction time. If the acid value is less than 120, it is considered that the carboxylation reaction is over. The second step: acidification and separation, use 30% HCl solution to neutralize to pH=4±0.5, raise the tem...
preparation Embodiment 1-1
[0038] The preparation of didodecanyl secondary amine ether (5EO) carboxylic acid shown in formula V
[0039]
[0040]A kind of preparation method of didodecanyl secondary amine ether (5EO) carboxylic acid, comprises the following steps: the first step: carboxylation reaction, earlier raw material 1.2mol didodecanyl secondary amine ether (5EO) and 1mol chlorine Sodium acetate was placed in the experimental device equipped with a stirrer, thermometer, water separator and condenser tube. The whole experimental device was inerted for 3 times, and finally nitrogen protection was introduced, the condenser was opened, and the temperature was raised to 40-90 °C, and 1.25 mol Sodium hydroxide is evenly divided into 5-10 parts, and added in batches and evenly within 8-20 hours of reaction time. If the acid value is less than 120, it is considered that the carboxylation reaction is over. The second step: acidification and separation, use 30% HCl solution to neutralize to pH = 4±0.5, ...
preparation Embodiment 1-2
[0042] The preparation of didodecanyl secondary amine ether (5EO) carboxylic acid shown in formula V
[0043]
[0044] A kind of preparation method of didodecanyl secondary amine ether (5EO) carboxylic acid, comprises the following steps: the first step: carboxylation reaction, earlier raw material 1.2mol didodecanyl secondary amine ether (5EO) and 1mol chlorine Sodium acetate was placed in an experimental device equipped with a stirrer, thermometer, water separator and condenser. The experimental device was inerted three times, and finally nitrogen protection was introduced, the condenser was opened, and the temperature was raised to 40-90°C. Sodium hydroxide is evenly divided into 5-10 parts, and added in batches and evenly within 8-20 hours of reaction time. If the acid value is less than 120, it is considered that the carboxylation reaction is over. The second step: acidification and separation, use 30% HCl solution to neutralize to pH = 4±0.5, raise the temperature to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com