Radix millettiae speciosae traditional Chinese medicine composition with kidney-tonifying and bone-reinforcing function and preparation method thereof
A technology for invigorating the kidney and strengthening the bones and composition, which is applied in the direction of drug combinations, medical preparations containing active ingredients, and pharmaceutical formulas, and can solve the problems of low extraction rate of active ingredients, long extraction time, and low equipment requirements.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
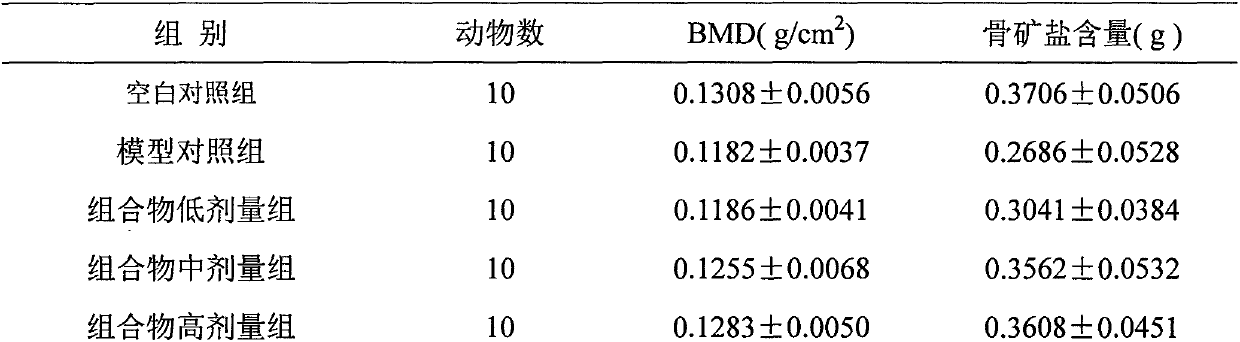
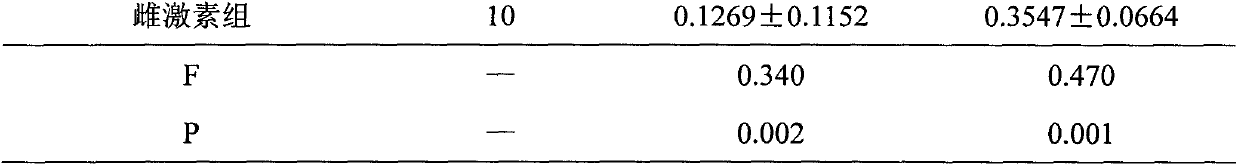
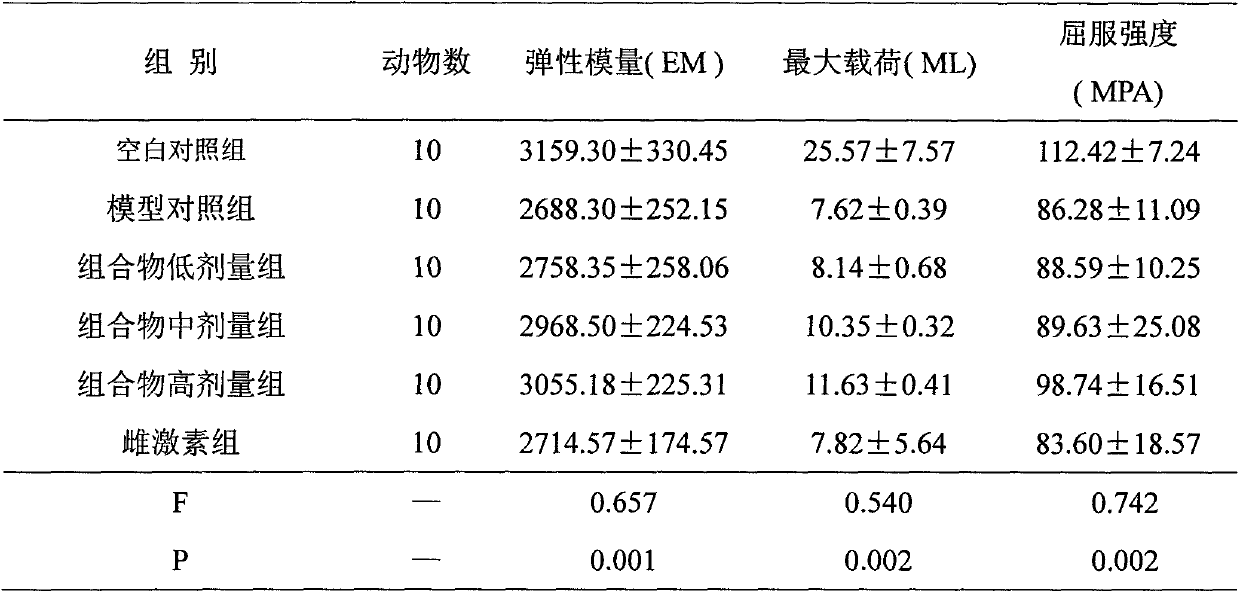
Image
Examples
Embodiment 1
[0033] prescription:
[0034] 10 parts of Niu Dali, 10 parts of Eucommia, 5 parts of Epimedium, 5 parts of Cuscuta, 5 parts of Salvia miltiorrhiza, 5 parts of Pueraria lobata, 5 parts of fructooligosaccharides, and 0.05 parts of calcium gluconate.
[0035] Preparation:
[0036] (1) Niu Dali, Eucommia, Epimedium, Cuscuta, Salvia Miltiorrhiza and Radix Puerariae were selected, dried, and weighed according to the weight ratio of the required raw materials;
[0037] (2) Put the weighed Chinese herbal medicine into an ultrafine pulverizer to pulverize, collect the micropowder (over 300 orders), and set aside;
[0038] (3) Put the micropowder obtained in step (2) into a Chinese medicine multifunctional extraction tank, add 8-10 times the amount of water, heat and extract 1-3 times, each time for 0.5-2h, filter, collect the filtrate, and merge;
[0039] (4) Heating and concentrating the filtrate into a thick extract, drying under reduced pressure into pieces (moisture ≤ 5%), pulver...
Embodiment 2
[0042] prescription:
[0043] Niu Dali 20 parts, Eucommia 20 parts, Epimedium 15 parts, Cuscuta 15 parts, Salvia miltiorrhiza 15 parts, Pueraria 10 parts, fructooligosaccharides 7 parts, calcium gluconate 1.0 parts.
[0044] Preparation:
[0045] (1) Niu Dali, Eucommia, Epimedium, Cuscuta, Salvia Miltiorrhiza and Radix Puerariae were selected, dried, and weighed according to the weight ratio of the required raw materials;
[0046] (2) Put the weighed Chinese herbal medicine into an ultrafine pulverizer to pulverize, collect the micropowder (over 300 orders), and set aside;
[0047] (3) Put the micropowder obtained in step (2) into a Chinese medicine multifunctional extraction tank, add 8-10 times the amount of water, heat and extract 1-3 times, each time for 0.5-2h, filter, collect the filtrate, and merge;
[0048] (4) Heating and concentrating the filtrate into a thick extract, drying under reduced pressure into pieces (moisture ≤ 5%), pulverizing, and collecting fine powder;...
Embodiment 3
[0051] prescription:
[0052] Niu Dali 30 parts, Eucommia 30 parts, Epimedium 25 parts, Cuscuta 25 parts, Salvia miltiorrhiza 25 parts, Pueraria radiata 15 parts, fructooligosaccharide 10 parts, calcium gluconate 2.0 parts.
[0053] Preparation:
[0054] (1) Niu Dali, Eucommia, Epimedium, Cuscuta, Salvia Miltiorrhiza and Radix Puerariae were selected, dried, and weighed according to the weight ratio of the required raw materials;
[0055] (2) Put the weighed Chinese herbal medicine into an ultrafine pulverizer to pulverize, collect the micropowder (over 300 orders), and set aside;
[0056] (3) Put the micropowder obtained in step (2) into a Chinese medicine multifunctional extraction tank, add 8-10 times the amount of water, heat and extract 1-3 times, each time for 0.5-2h, filter, collect the filtrate, and merge;
[0057] (4) Heating and concentrating the filtrate into a thick extract, drying under reduced pressure into pieces (moisture ≤ 5%), pulverizing, and collecting fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com