Injection preparation of epidermal growth factor receptor monoclonal antibody
A technology of epidermal growth factor and monoclonal antibody, which is applied in the direction of antibodies, anti-inflammatory agents, skin diseases, etc., can solve the problems of no reports, etc., and achieve good stability, good solubility and stability, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The preparation of embodiment 1 antibody
[0051] For the sequence information and preparation method of the antibody, please refer to the antibody BA03 disclosed in Chinese Patent Application Publication CN103772504A. details as follows:
[0052] The heavy chain variable region sequence of the antibody is:
[0053] QVQLQESGPGLVKPSETLSLTCTVSGFSLSNYDVHWVRQAPGKGLEWLGVIWSGGNTDYNTPFTSRLTISVDTSKNQFSLKLSSVTAADTAVYYCARALDYYDYEFAYWGQGTLVTVSS (SEQ ID NO: 1)
[0054] The light chain variable region sequence of the antibody is:
[0055] EIVLTQSPDFQSVTPKEKVTITCRASQSIGTNIHWYQQKPDQSPKLLIKYASESISGIPSRFSGSGSGTDFLTINSLEAEDAATYYCQQNNEWPTSFGQGTKLEIK (SEQ ID NO: 2)
[0056] (1) According to the heavy chain variable region sequence and the light chain variable region sequence of the antibody, respectively design and synthesize PCR primer oligonucleotide fragments encoding the heavy chain and light chain variable region sequences, and introduce the required restriction enzymes during synt...
Embodiment 2
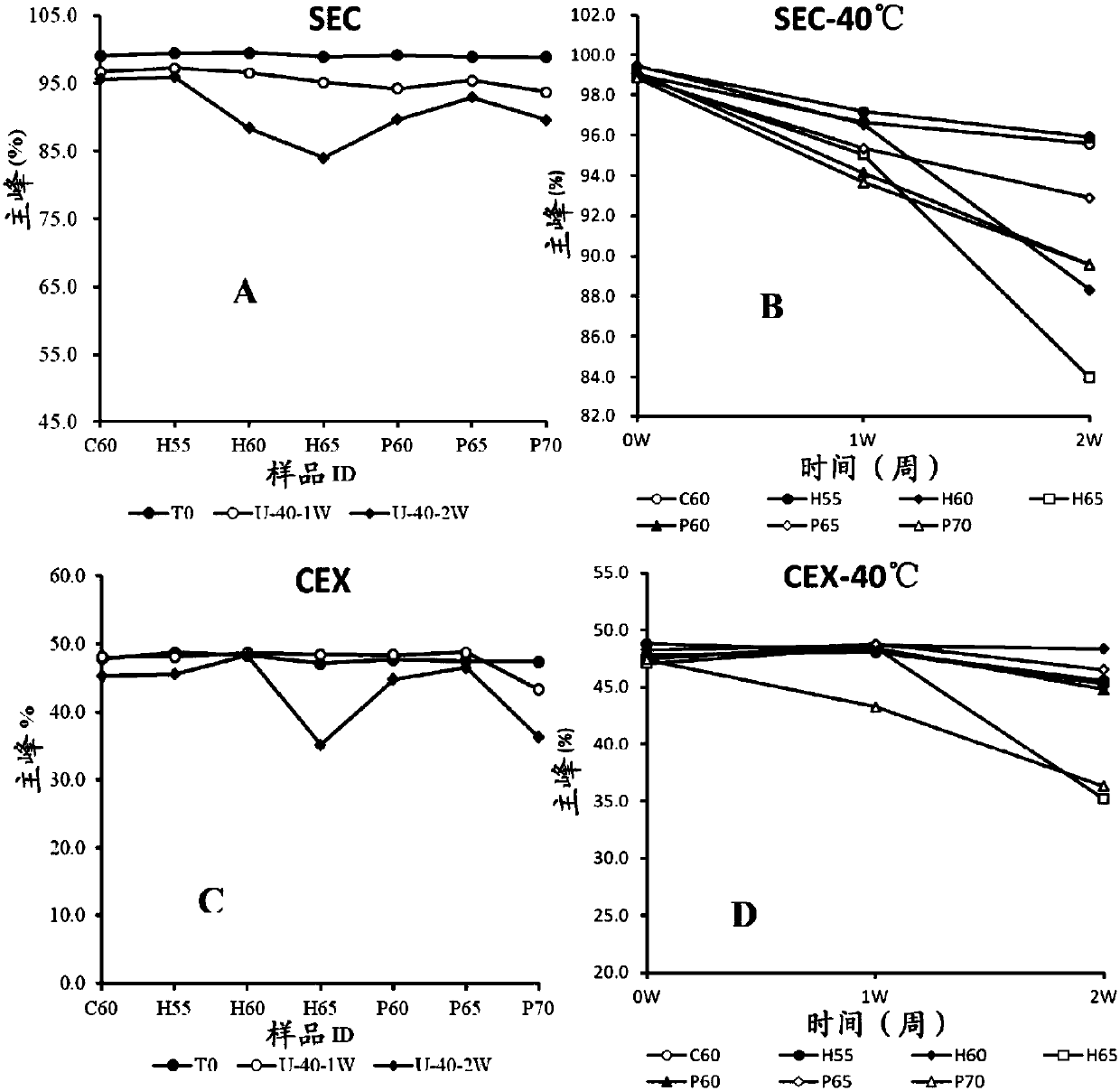
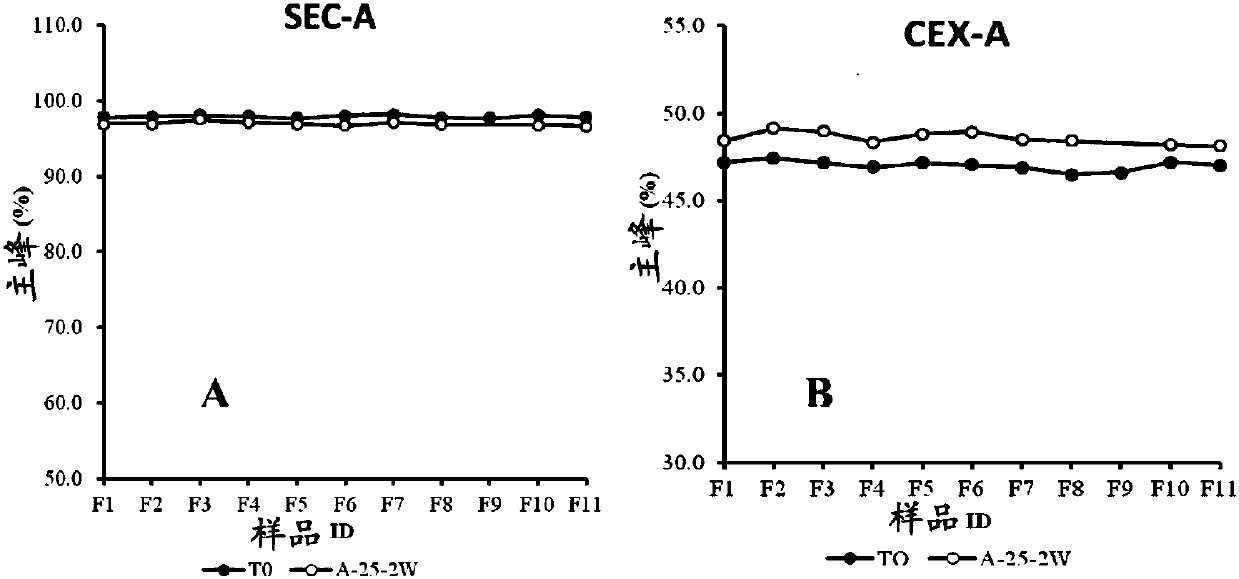
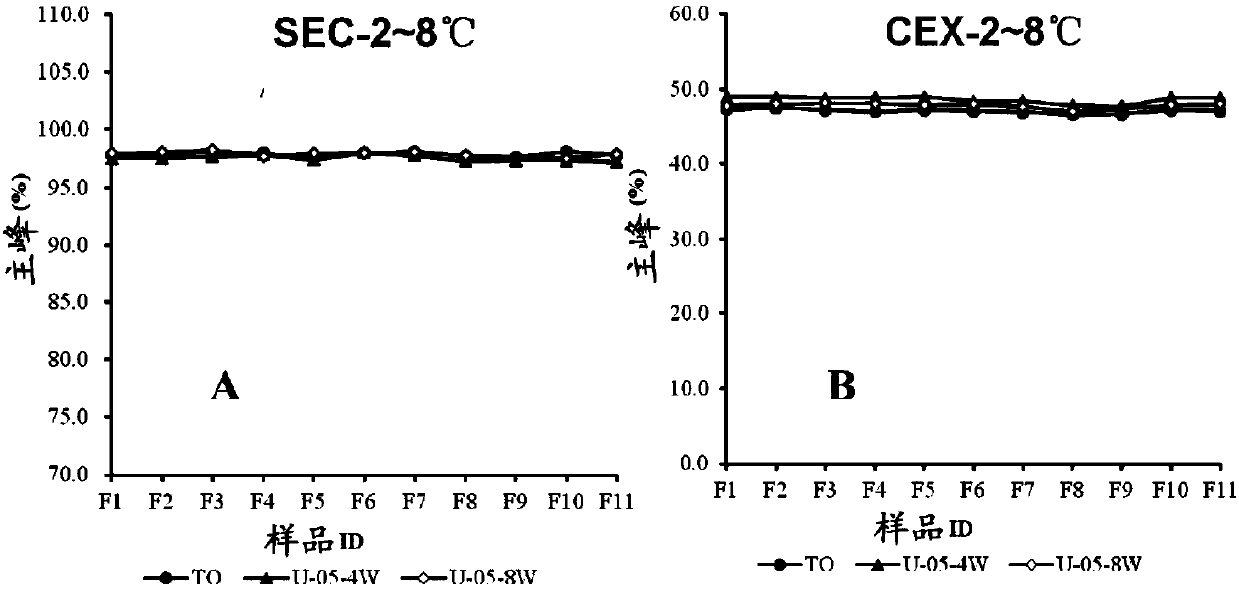
[0067] Example 2 Screening of buffer and pH combination in antibody BA03 injection preparation
[0068] 1) The purpose of this example is to screen the pH and buffer system suitable for antibody BA03. To this end, we designed 7 buffer systems, respectively 20mM citric acid system (containing citric acid and sodium citrate with a total concentration of 20mM), pH 6.0; 20mM histidine system (containing a total concentration of 20mM histidine and histidine hydrochloride), pH 5.5, 6.0, 6.5; and 20mM phosphoric acid system (containing a total concentration of 20mM phosphoric acid, sodium dihydrogen phosphate and disodium hydrogen phosphate), pH 6.0, 6.5, 7.0. Since the 20mM pH6.5 histidine system and the 20mM pH6.5 phosphoric acid system precipitated during the ultrafiltration process, the protein precipitated out, so 0.05% PS80 and 0.87% sodium chloride solution were added to redissolve the protein. See Table 1 for details. In each buffer system, the antibody concentration was 10...
Embodiment 3
[0089] Example 3 Screening of other adjuvants in the antibody BA03 injection preparation
[0090] 1) Screening program for excipients
[0091] According to the buffer system screening results, we have selected 20mM histidine buffer system (total concentration is 20mM histidine and histidine hydrochloride, pH5.5) and 20mM citric acid buffer system (total concentration is 20mM citric acid and sodium citrate, pH6.0) as the buffer system designed for the prescription. In addition, in the previous buffer screening, we did not investigate the pH below 5.5. In the formulation screening, we added the 20mM histidine system with pH 4.5 and pH 5.0 respectively (total concentration of 20mM histidine and histidine Amino acid hydrochloride) to ensure that the final pH selected is the optimum pH. The selection and amount and range of auxiliary materials are 0-5% sucrose, 0-5% trehalose, 0-0.05% Tween 80, 0-0.87% sodium chloride and 0-2% glycine. The excipients and prescription screening s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com