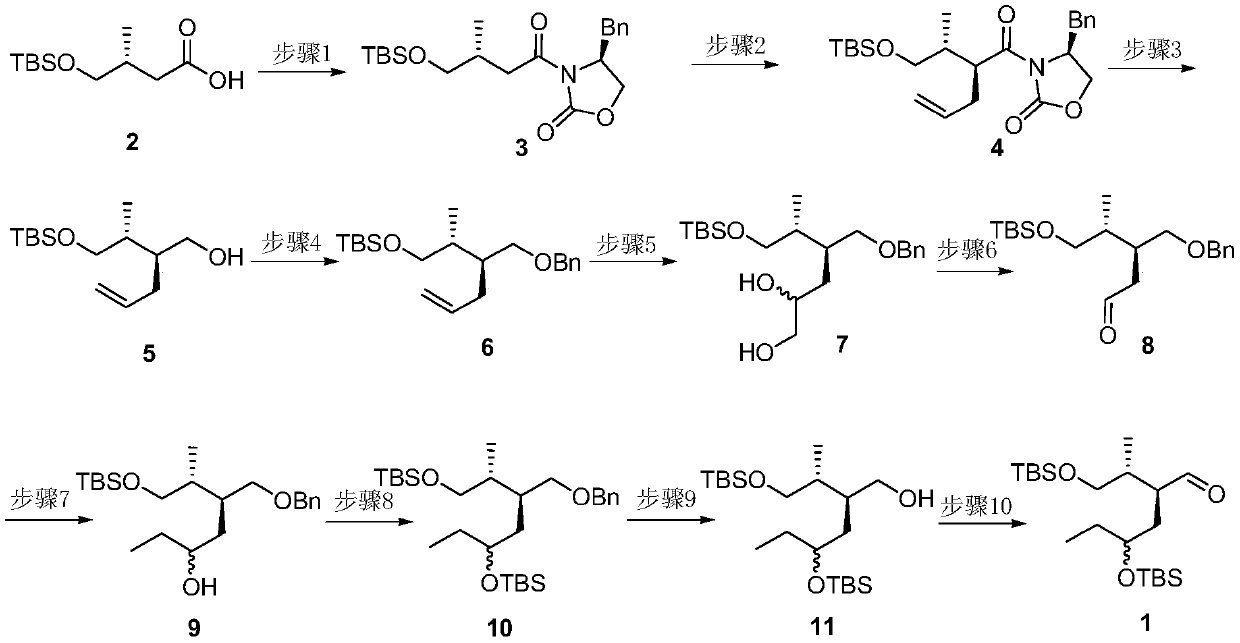
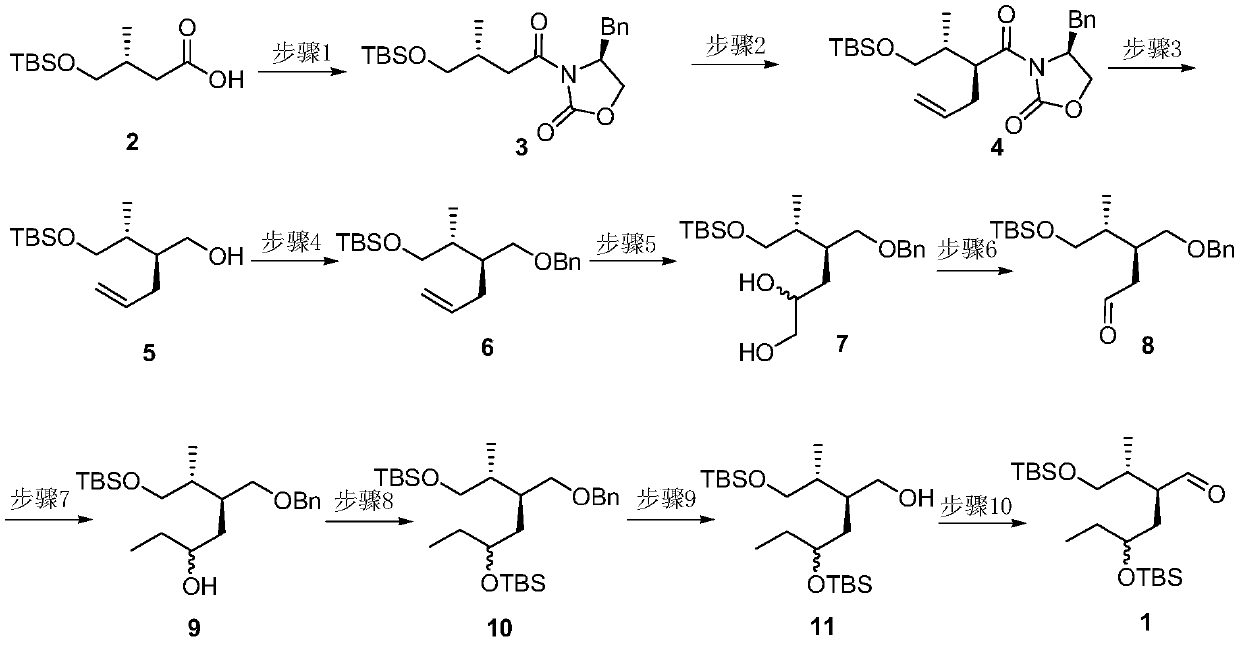
A method for preparing a key chiral fragment of artemisinic acid
A key technology of artemisinic acid, which is applied in the field of preparation of a key chiral fragment of artemisinic acid, can solve the problems of asymmetric synthesis of artemisinic acid that have not been reported yet, and achieve a simple route, high yield, and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Synthesis of compound 3(S)-4-benzyl-3-((R)-4-(tert-butyldimethylsilyloxy)-3-methylbutyryl)-2-oxazolidinone:
[0046] Under nitrogen protection, compound 2(R)-4-((tert-butyldimethylsilyl)oxy)-3-methylbutanoic acid (47.5g, 204mmol) was dissolved in 800ml dry tetrahydrofuran (THF ), add triethylamine (56ml, 408mmol) and pivaloyl chloride (30ml, 243mmol) at -78°C, react for 2h, then add lithium chloride (25.9g, 612mmol), (S)-4-benzyl -2-oxazolidinone (36.2g, 204mmol), reacted for 18 hours, then turned off the low-temperature refrigeration, slowly raised the temperature, stirred overnight, quenched the reaction system with water, removed THF by rotary evaporation, then extracted 3 times with ethyl acetate, saturated Washed once with brine, dried over anhydrous magnesium sulfate, filtered, concentrated, and column chromatographed to obtain compound 3(S)-4-benzyl-3-((R)-4-(tert-butyldimethylsiloxane yl)-3-methylbutyryl)-2-oxazolidinone (72.8 g, 92%);
[0047] Synthesis of co...
Embodiment 2
[0071] Synthesis of compound 3(S)-4-benzyl-3-((R)-4-(tert-butyldimethylsilyloxy)-3-methylbutyryl)-2-oxazolidinone:
[0072] Under nitrogen protection, compound 2(R)-4-((tert-butyldimethylsilyl)oxy)-3-methylbutanoic acid (47.5g, 204mmol) was dissolved in 800ml dry tetrahydrofuran (THF ), add triethylamine (56ml, 408mmol) and pivaloyl chloride (30ml, 243mmol) at -78°C, react for 2h, then add zinc chloride (83.4g, 612mmol), (S)-4-benzyl -2-oxazolidinone (36.2g, 204mmol), reacted for 30min, then turned off the low-temperature refrigeration, slowly raised the temperature, stirred overnight, quenched the reaction system with water, removed THF by rotary evaporation, then extracted 3 times with ethyl acetate, saturated salt Washed once with water, dried over anhydrous magnesium sulfate, filtered, concentrated, and column chromatographed to obtain compound 3(S)-4-benzyl-3-((R)-4-(tert-butyldimethylsilyloxy )-3-methylbutyryl)-2-oxazolidinone (72.8 g, 92%);
[0073] Synthesis of compo...
Embodiment 3
[0096] Synthesis of compound 3(S)-4-benzyl-3-((R)-4-(tert-butyldimethylsilyloxy)-3-methylbutyryl)-2-oxazolidinone:
[0097] Under nitrogen protection, compound 2(R)-4-((tert-butyldimethylsilyl)oxy)-3-methylbutanoic acid (47.5g, 204mmol) was dissolved in 800ml dry tetrahydrofuran (THF ), add triethylamine (56ml, 408mmol) and pivaloyl chloride (30ml, 243mmol) at -78°C, react for 2h, then add magnesium chloride (25.9g, 612mmol), (S)-4-benzyl-2 -Zoxazolidinone (36.2g, 204mmol), reacted for 25 hours, then turned off the low-temperature refrigeration, slowly raised the temperature, stirred overnight, quenched the reaction system with water, removed THF by rotary evaporation, then extracted 3 times with ethyl acetate, and saturated saline Wash once, dry over anhydrous magnesium sulfate, filter, concentrate, and column chromatography to obtain compound 3(S)-4-benzyl-3-((R)-4-(tert-butyldimethylsilyloxy) -3-methylbutyryl)-2-oxazolidinone (72.8 g, 92%);
[0098] Synthesis of compound ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com