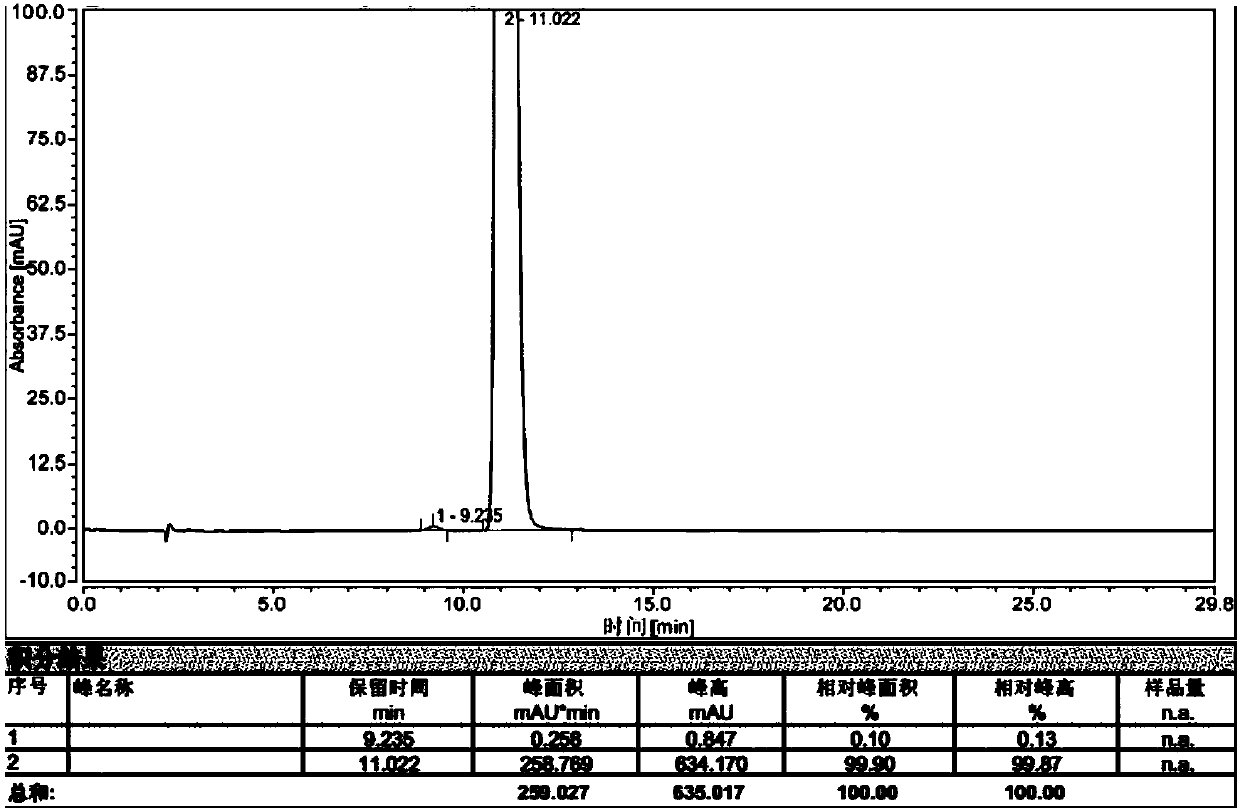
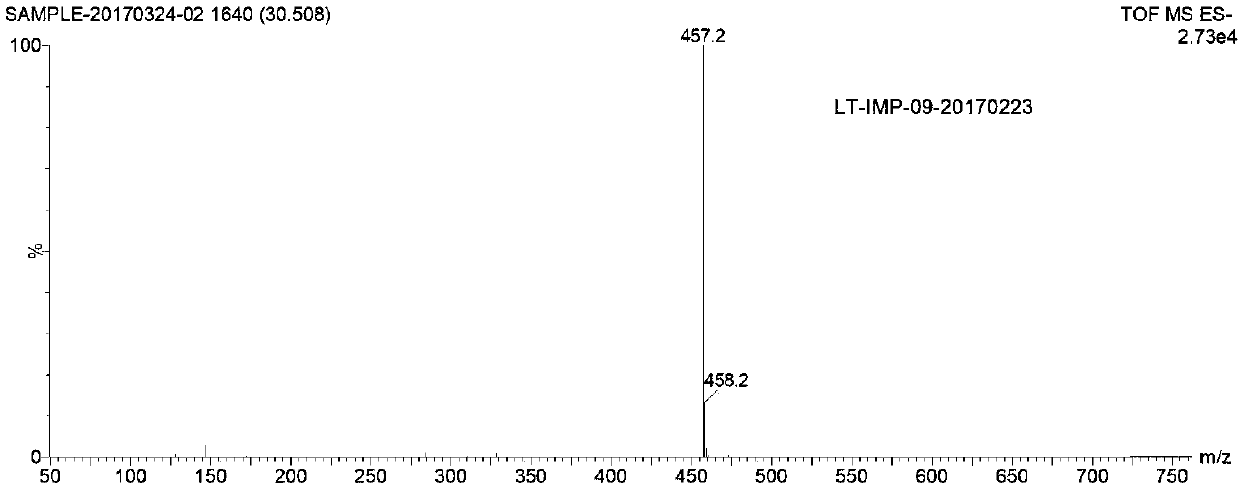
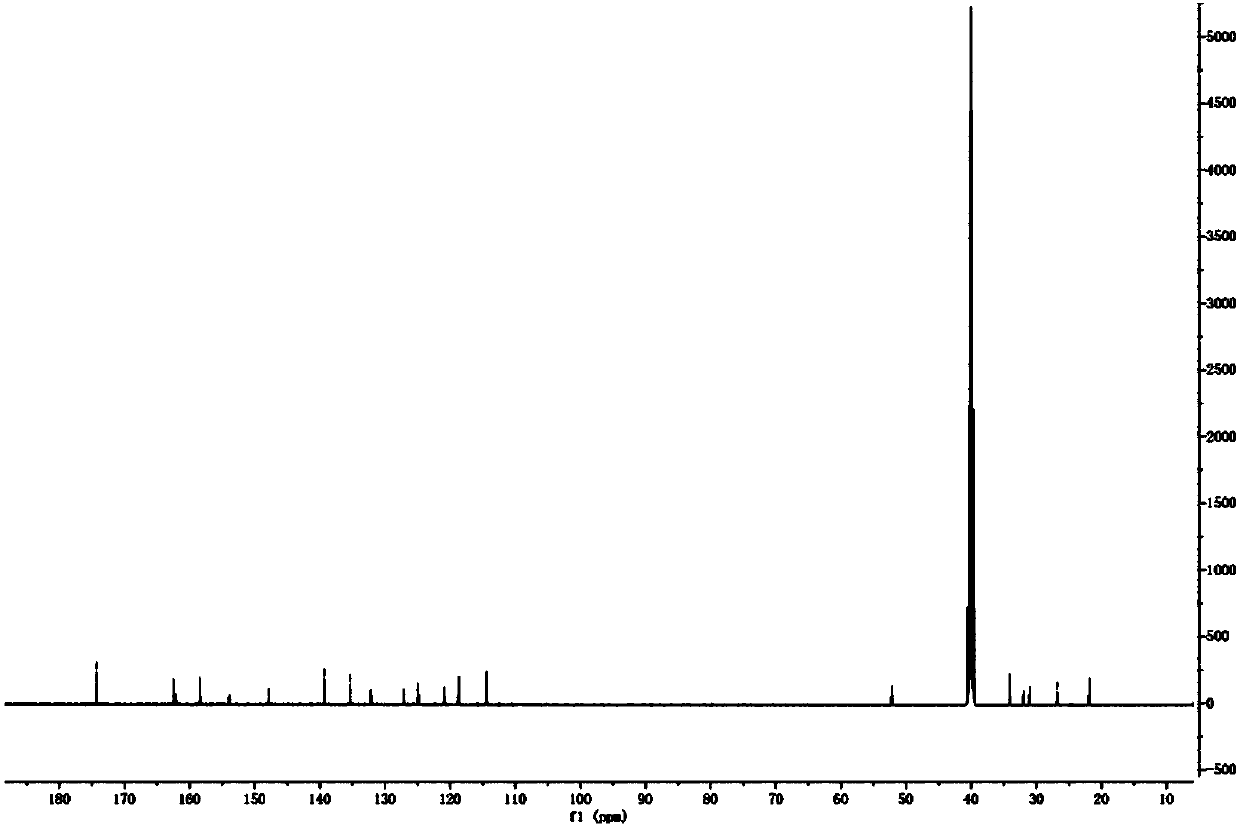
Raltitrexed related substance C as well as preparation and application thereof
A technology of raltitrexed, related substances, applied in the field of medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Preparation of N-(5-methylamino-2-thiophenoyl)-L-glutamic acid diethyl ester
[0039] Add 10g of 5-nitro-2-thiophenecarboxylic acid and 20ml of thionyl chloride into the reaction flask, raise the temperature and reflux for 3 hours, transfer the reaction solution to a single-necked flask, concentrate until no liquid flows out, and dissolve it in 30ml of dichloromethane for later use . Add 13.8g of diethyl L-glutamate, 20.6g of triethylamine, and 50ml of dichloromethane into a 150ml three-neck flask, stir and cool down to 0-5°C, and slowly add the dichloromethane solution of the concentrate dropwise. After dropping, keep warm for 1 hour. After the reaction, wash with 100 ml of 2 mol / L hydrochloric acid and 100 ml of saturated sodium bicarbonate solution successively. The organic phase was separated, dried over anhydrous sodium sulfate, and concentrated to obtain 16.53 g of N-(5-nitro-2-thiophenoyl)-L-glutamic acid diethyl ester with a yield of 79.9%.
[0040]...
Embodiment 2
[0042] Example 2 Preparation of Intermediate 2
[0043] Add 8.02g of N-(5-methylamino-2-thiophenoyl)-L-glutamic acid diethyl ester, 8g of Boc anhydride, and 100ml of absolute ethanol into the reaction flask, stir and heat up to reflux, and continue the reaction for 2 hours. TLC monitored the reaction to the end point, distilled off ethanol, added 100ml of ethyl acetate, extracted 150ml of purified water in two equal parts, removed the water phase, added 20g of anhydrous magnesium sulfate to the organic phase, stirred and dried for 1 hour, and suction filtered to obtain the filtrate. The solvent was distilled off to obtain 10.29 g of an oily yellow liquid (Intermediate 2), with a yield of 96%.
Embodiment 3
[0044] Example 3 Preparation of Intermediate 3
[0045] 10.29g intermediate 2, 3.26g AlCl 3, 15.62g of 6-bromomethyl-3,4-dihydro-2-methyl-quinazolin-4-one and 90ml of DMF were added to the reaction flask, replaced with nitrogen three times, slowly heated to 100°C, and kept for 12 hours. Cool to room temperature, add 100ml of purification and stir, wash with 150ml of ethyl acetate three times evenly, discard the lower aqueous phase, add 30g of anhydrous magnesium sulfate to the organic phase, stir and dehydrate for 1 hour, filter with suction to obtain the filtrate, distill the filtrate to obtain an oil things.
[0046] Add 200ml of ethyl acetate to the obtained oil, mix the sample with 1.2 times the mass of silica gel, spin dry to make a sandy shape, dry the sample, add 5 times the mass of silica gel and two layers of filter paper to the upper layer of the sample layer, and use the eluent ethyl acetate Esters were eluted and TLC monitored the start and end of eluate collecti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com