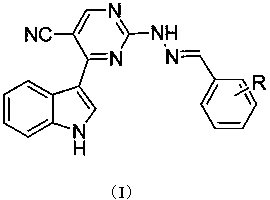
Indolylpyrimidine ring-containing hydrazone compound, and preparation method and application thereof
A technology of indole pyrimidine ring hydrazones and compounds, which is applied in the field of a class of indole pyrimidine ring hydrazone compounds and their preparation and application, can solve the problems that no one has synthesized hydrazone compounds, achieve good tumor performance, and improve self Antitumor activity, effect of enhancing antitumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] a) Synthesis of 2-(2-benzylhydrazino)-4-(1H-indole-3-carbonyl)-pyrimidine-5-carbonitrile.
[0047]
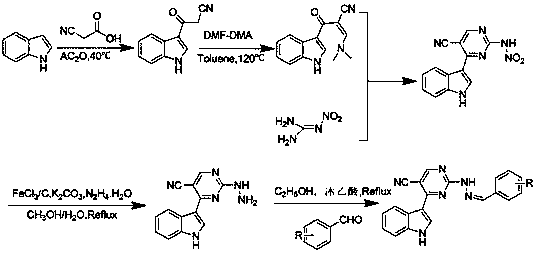
[0048] Step 1: Synthesis of 3-(1H-indole-3-)-3-oxopropanecyanide
[0049] Weigh 11.72 g of indole and 8.51 g of cyanoacetic acid, put them into a 500 mL pear-shaped bottle, add 100 mL of acetic anhydride, adjust to 40°C, react for 4 hours, cool and filter, wash with methanol, and dry to obtain 15.66 g of white powder. The rate is 85%, and the melting point is 240~241°C.
[0050] The second step: Synthesis of 3-dimethylamino-2-(1H-indole-3-carbonyl)-acrylonitrile
[0051] Add 20 mL of toluene dissolved with 6.0g of DMF-DMA into 150 mL of toluene dissolved with 9.2g of 3-(1H-indole-3-)-3-oxopropanecyanide, heat to 120°C, react for 3h, and cool After suction filtration, a yellow solid was obtained, which was recrystallized from ethanol to obtain 10.82 g of a yellow powder, with a yield of 90.2% and a melting point of 160-161°C.
[0052] Step 3: Synthesis of N-(5-cyano...
Embodiment 2
[0060] b) Preparation of 2-(2-(4-methylbenzyl)hydrazino)-4-(1H-indole-3-carbonyl)-pyrimidine-5-carbonitrile.
[0061] Prepared according to the method and conditions of Example 1. The first, second, third, and fourth steps are the same as in Example one, except that the fifth step adds 0.50 g of 2-hydroxybenzaldehyde, and the target product yield is 41.7%.
[0062]
[0063] Molecular formula: C 21 h 14 N 6 ; Yield: 85.7%; Off-white powdery solid; m.p.>250℃; ESI-MS: 353.2,[M+H] +,375.1 [M+Na] +; IR (KBr, cm-1) v: 3283, 3207, 2978, 2220,1570, 1528,1506, 1487, 1447, 1416, 1259, 1144, 1096, 810, 795, 754; 1H NMR(500 MHz,DMSO) δ (ppm) (TMS as internal standard): 12.11 (s, 1H, indole-NH), 11.88 (s, 1H, NH), 9.33(s, 1H, Pyr-H), 8.78 (s, 1H), 8.63 (s, 1H, Ph-H), 8.30 ( 13C NMR(125 MHz, DMSO) δ (ppm): 163.6, 163.5, 159.9, 144.8, 140.0, 137.0, 131.1, 130.0, 127.2, 123.6, 121.9, 119.9, 112.8, 91.3, 21.6.
Embodiment 3
[0065] c) Preparation of 2-(2-(4-fluorobenzyl)hydrazino)-4-(1H-indole-3-carbonyl)-pyrimidine-5-carbonitrile.
[0066] Prepared according to the method and conditions of Example 1. The first, second, third, and fourth steps are the same as in Example 1, except that 0.50 g of 2-fluorobenzaldehyde is added in the fifth step, and the yield of the target product is 75.8%.
[0067]
[0068] Molecular formula: C 20 h 13 FN 6 ; Yield: 72.5%; Earthy yellow powdery solid; m.p.>250℃; ESI-MS: 357.1[M+H] +, 379.1 [M+Na] +; IR (KBr, cm-1) v: 3308, 1H NMR (500 MHz, DMSO) (pp TMS as internal standard): 12.12 (s,1H, indole-NH), 9.31(s, 1H, Pyr-H), 8.80 (s, 1H), 8.6 (s, 1H, NCH), 8.32 (s, 1H, Ph-H), 7.91 (s,2H, Ph-H), 7.60 (d, 1H, J=8.6Hz, Ph-H), 7.42 (s, 3H, Ph-H), 7.31(d, 1H, J =8.0Hz, Ph-H); 13 C NMR (125 MHz, DMSO) δ (ppm): 164.4, 163.6,162.4, 159.8,143.7, 136.9, 131.1, 129.1, 124.7, 123.7, 122.0, 119.8, 116.57,1 , 112.8, 91.3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com