Zinc compounds adopting 2,6-diacetyl pyridine thiosemicarbazone as a ligand, a synthetic method of the compounds and applications of the compounds
A technology of diacetylpyridine thiosemicarbazide, zinc compound, applied in zinc organic compound, drug combination, organic chemistry and other directions, can solve the problem of no antitumor effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: the synthesis of C1 zinc compound
[0043] The specific synthesis method is:
[0044] (1) Dissolve 10mmol of 2,6-diacetylpyridine in 20mL of ethanol, stir at 60°C for 15min to obtain a solution, drop the above solution into 20mL of ethanol solution added with 10mmol of thiosemicarbazide, Reflux and stir at 60°C for 12 hours, cool to room temperature, pour into a beaker for volatilization, filter the light yellow crystals obtained above and wash with absolute ethanol 3 times to obtain the ligand (L1);
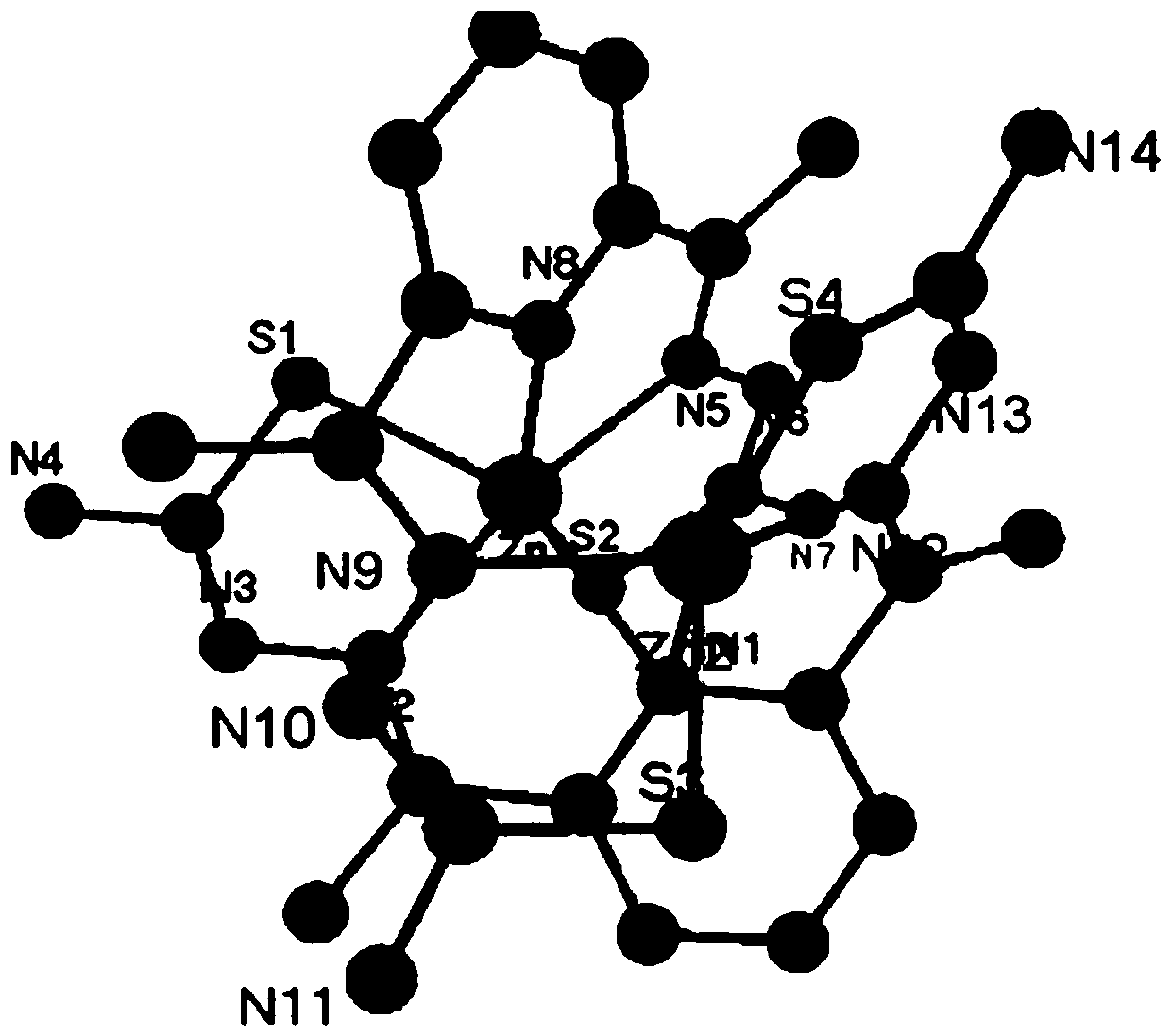
[0045] (2) will contain ZnCl 2 Add (136.30mg, 1mmol) 20mL methanol solution dropwise to 10mL ethanol solution containing 1mmol 2,6-diacetylpyridine thiosemicarbazone ligand, reflux and stir at 60°C for 2h, filter the reacted solution into a 50mL beaker , and sealed with plastic wrap, needle-punched 10 holes and volatilized at room temperature for several days to obtain light yellow crystals (C1), whose single crystal structure is as follows figure 1 shown...
Embodiment 2
[0047] Embodiment 2: the synthesis of C2 zinc compound
[0048] The specific synthesis method is:
[0049] (1) Dissolve 10mmol of 2,6-diacetylpyridine in 20mL of ethanol, stir at 60°C for 15min to obtain a solution, drop the above solution into 20mL of ethanol with 10mmol of 4-methylthiosemicarbazide In the solution, reflux and stir at 60°C for 12 hours, cool to room temperature, pour into a beaker for volatilization, filter the light yellow crystals obtained above, and wash with absolute ethanol 3 times to obtain the ligand (L2);
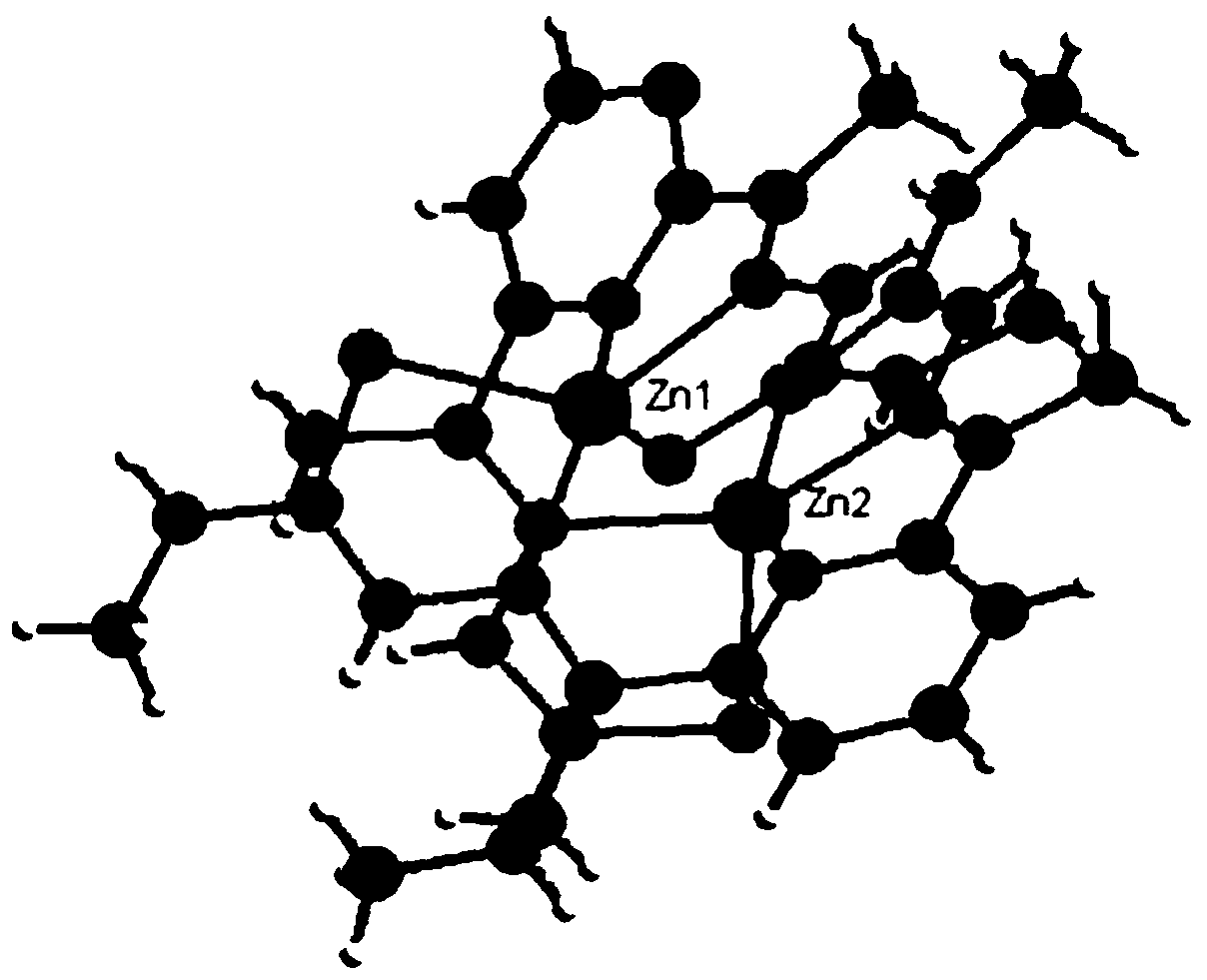
[0050] (2) will contain ZnCl 2 (136.30 mg, 1 mmol) 20 mL of methanol solution was added dropwise to 10 mL of ethanol solution containing 1 mmol of the above-mentioned L2 ligand, stirred at reflux at 60°C for 2 h, filtered the reacted solution into a 50 mL beaker, sealed with plastic wrap, and pricked with a needle 10 holes were volatilized at room temperature for several days to obtain pale yellow crystals (C2), whose single crystal structure is...
Embodiment 3
[0052] Embodiment 3: the synthesis of C3 zinc compound
[0053] The specific synthesis method is:
[0054] (1) Dissolve 10mmol of 2,6-diacetylpyridine in 20mL of ethanol, stir at 60°C for 15min to obtain a solution, add the above solution dropwise to 20mL with 10mmol of 4,4-diethylthio In the ethanol solution of semicarbazide, reflux and stir at 60°C for 12 hours, cool to room temperature, pour into a beaker to volatilize, filter the light yellow crystals obtained above, wash 3 times with absolute ethanol, and dry to obtain the ligand (L3 ),;
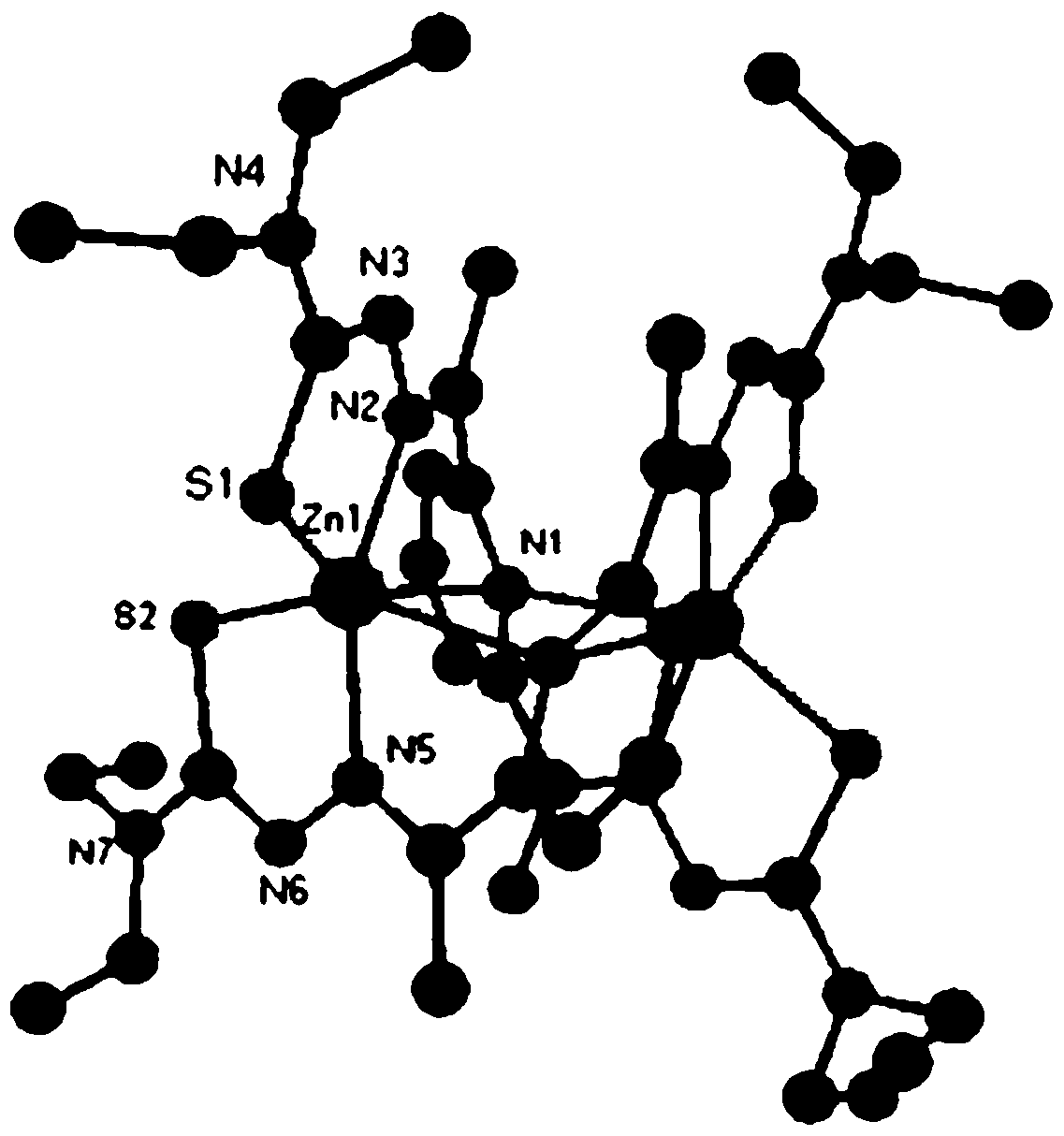
[0055] (2) will contain ZnCl 2 (136.30 mg, 1 mmol) 20 mL of methanol solution was added dropwise to 10 mL of ethanol solution containing 1 mmol of the above-mentioned L3 ligand, stirred at reflux at 60°C for 2 h, filtered the reacted solution into a 50 mL beaker, sealed with plastic wrap, and pricked with a needle Ten holes were volatilized at room temperature for several days to obtain orange-yellow crystals (C3), whose single crys...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com