Organic electroluminescent material and organic luminescent device thereof
An electroluminescent material and organic technology, applied in organic light-emitting devices, light-emitting materials, materials of organic semiconductor devices, etc., can solve the problems of low luminous efficiency, high driving voltage, unsatisfactory luminous performance, etc., and achieve high luminous efficiency, The effect of low driving voltage and strong carrier transport capability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
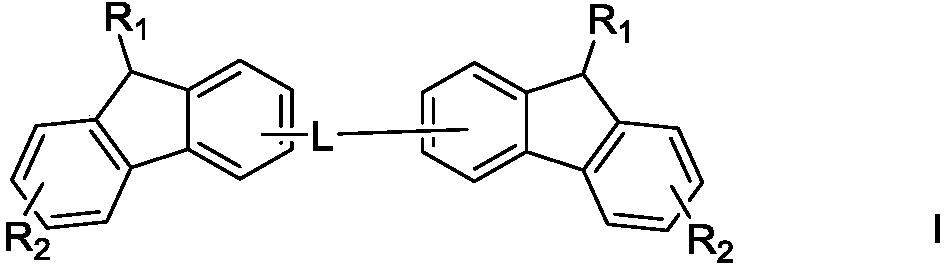
[0043] The preparation method of a kind of organic electroluminescent material described in the present invention, comprises that shown raw material generates a kind of organic electroluminescent material shown in formula I by following route reaction:
[0044]
[0045] Among them, R 1 , R 2 Independently selected from substituted or unsubstituted C6-C50 aryl, substituted or unsubstituted C6-C50 arylamine, substituted or unsubstituted C6-C50 aryl ether, substituted or unsubstituted C3-C50 heteroaryl A sort of;
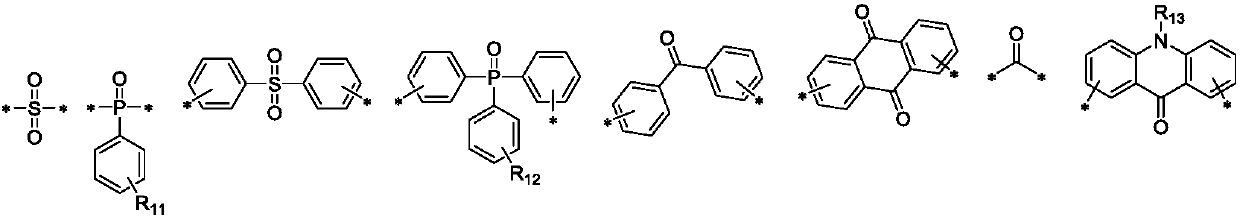
[0046] L is selected from one of substituted or unsubstituted sulfone groups, substituted or unsubstituted phosphinyl groups, substituted or unsubstituted carbonyl groups, and substituted or unsubstituted C3-C50 heteroaryl groups;
[0047] The heteroatom in the heteroaryl group is at least one of B, N, O, S, Si or P.
[0048] The present invention also provides an organic light emitting device, and the organic light emitting device may be an organic light emittin...
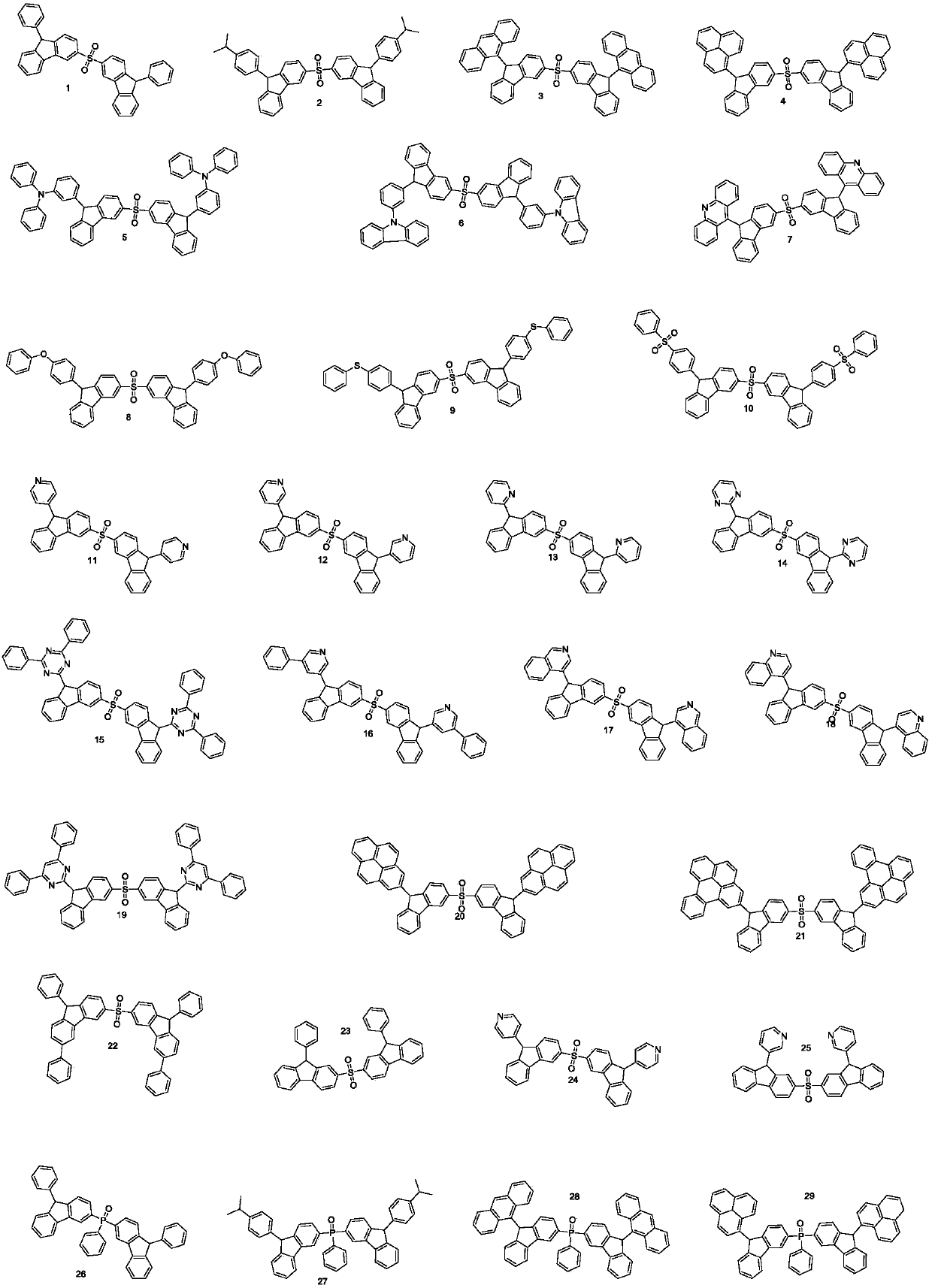
Embodiment 1
[0049] Embodiment 1: the preparation of compound 1
[0050]
[0051] Step1. Take 100mmol 3-bromo-9H-fluorenone, add 1 equivalent of 3-thiol-9H-fluorenone, 300mmol potassium tert-butoxide, 1mmol Pd2 (dba) 3 , toluene, replaced with argon three times, added 1 mmol of tri-tert-butylphosphine, replaced with argon three times again, reacted at reflux temperature for 10 h, passed the crude product through a silica gel column, and obtained 72 mmol of compound 1-1.
[0052] Step2. Take 72 mmol of compound 1-1, dissolve in DCM, cool to 0 degrees Celsius, add 2 equivalents of m-CPBA, gradually warm up to room temperature, stir and react for 6 hours, after the reaction is completed, wash with saturated anhydrous sodium bisulfite , the organic phase was dried and spin-dried, and the obtained product was passed through a silica gel column to obtain 1-250 mmol of the target product.
[0053] Step3. Take 50 mmol 1-2, add toluene to dissolve, add 2.5 equivalents of 4-methylbenzenesulfonyl...
Embodiment 2
[0054] Embodiment 2: the preparation of compound 6
[0055] Synthetic method is the same as embodiment 1, and phenylboronic acid is replaced as:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com