A kind of composite phase change material and preparation method thereof
A composite phase change material and a technology for phase change materials, which are applied in the field of composite phase change materials, can solve the problems of low subcooling and reduce phase separation problems, and achieve the effects of slowing down phase separation, reducing phase separation problems and having broad application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
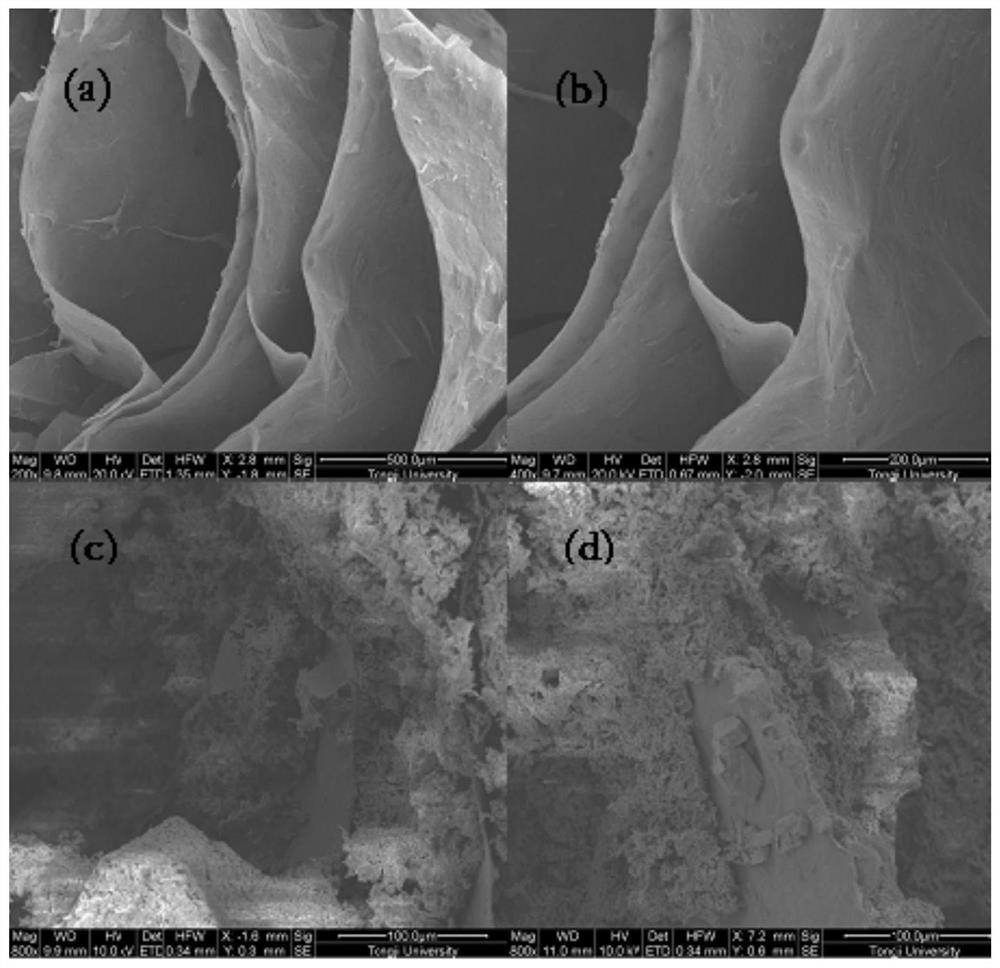
[0032] The graphite oxide solution was prepared by the improved Hummers method in the laboratory. After diluting the graphite oxide solution to 5 mg / ml and ultrasonicating for 1 h, 60 mL was taken in a hydrothermal kettle, kept in a vacuum oven at 180 °C for 16 h, cooled to room temperature, and obtained 3D graphene hydrogels. The hydrogel was pre-frozen for 2 hours, kept in a vacuum freeze dryer at a vacuum degree of ≤100 Pa, and freeze-dried for 24 hours to obtain a three-dimensional sleeve-shaped graphene aerogel.
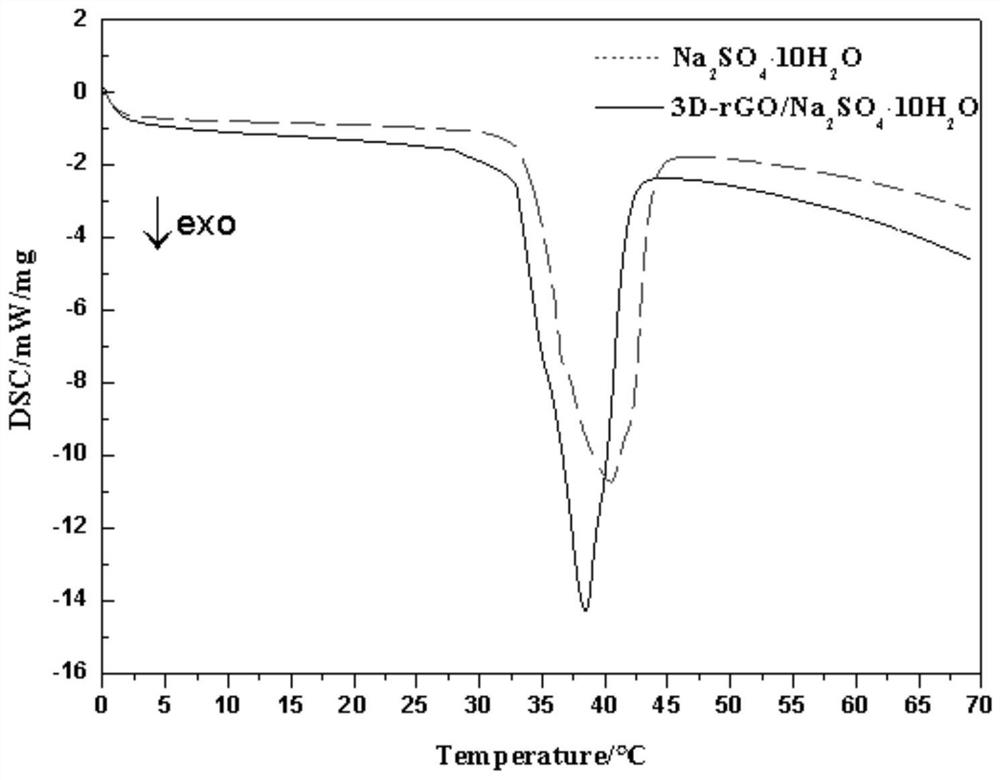
[0033] excess Na 2 SO 4 10H 2 O and three-dimensional graphene are placed in a beaker, and Na 2 SO 4 10H 2 The amount of O satisfies in Na 2 SO 4 10H 2 O can fully immerse 3D graphene after melting. Seal the beaker with plastic wrap and place it in a vacuum drying oven. 2 SO 4 10H 2 O fully melted. Take out the beaker, and use a syringe needle to poke about 30 to 60 holes in the insurance film, and the pores are evenly distributed.
[0034] The bea...
Embodiment 2
[0036] Graphite oxide solution was prepared in laboratory by improved Hummers method. Dilute the above-prepared graphite oxide solution to 8 mg / ml and sonicate for 2 hours, take 60 ml and place it in a hydrothermal kettle, keep it in a vacuum oven at 180°C for 24 hours, and cool to room temperature to obtain a three-dimensional graphene hydrogel. The hydrogel was pre-frozen for 3 hours, kept in a vacuum freeze dryer at a vacuum degree of ≤100 Pa, and freeze-dried for 36 hours to obtain a three-dimensional sleeve-shaped graphene aerogel.
[0037] Na 2 SO 4 10H 2 O and three-dimensional graphene are placed in a beaker, and Na 2 SO 4 10H 2The amount of O satisfies in Na 2 SO 4 10H 2 O can fully immerse 3D graphene after melting. Seal the beaker with plastic wrap and place it in a vacuum drying oven. 2 SO 4 10H 2 O fully melted. Take out the beaker, and use a syringe needle to pierce the insurance film about 50 times, and the pores are evenly distributed.
[0038] Th...
Embodiment 3
[0040] Graphite oxide solution was prepared in laboratory by improved Hummers method. Dilute the graphite oxide solution prepared above to 7 mg / ml and sonicate for 2 hours, take 45 ml and place it in a hydrothermal kettle, keep it in a vacuum oven at 160°C for 12 hours, and cool to room temperature to obtain a three-dimensional graphene hydrogel. The hydrogel was pre-frozen at -30°C for 4 hours, kept in a vacuum freeze dryer at a vacuum degree of ≤100 Pa, and freeze-dried at -30°C for 24 hours to obtain a three-dimensional sleeve-shaped graphene aerogel.
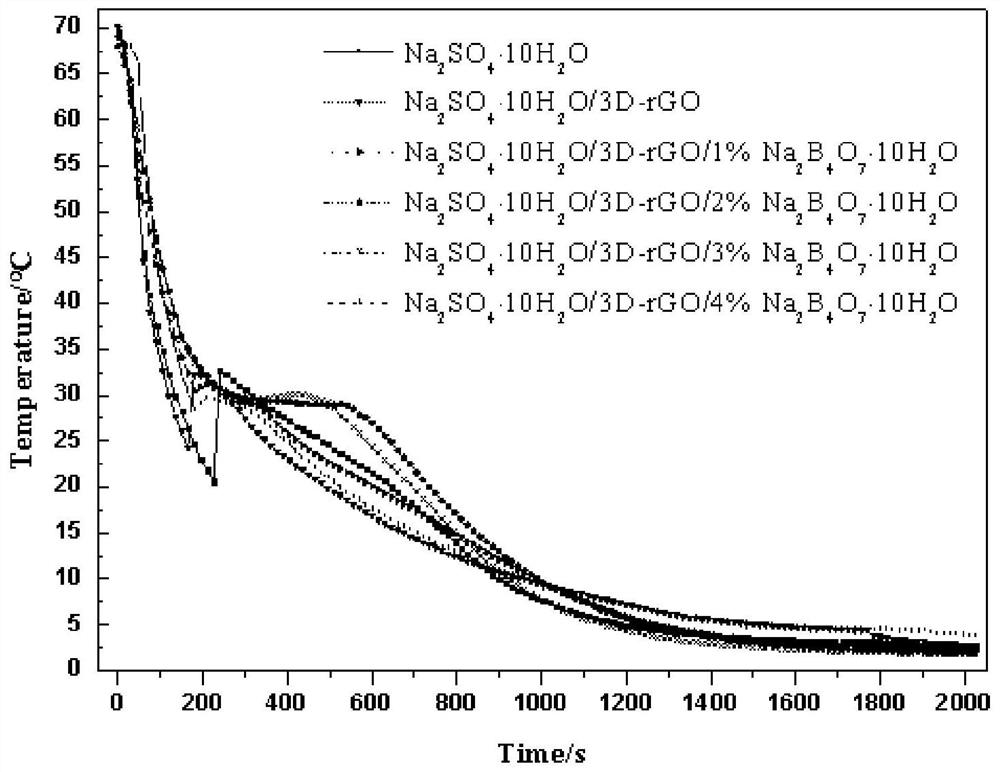
[0041] Borax, Na 2 SO 4 10H 2 O was mixed evenly at a mass ratio of 1:100, 1:50, 1:33, and 1:25, and the mixture and three-dimensional graphene were placed in a beaker, and the amount of the mixture added was sufficient to completely submerge the three-dimensional graphene after melting. Seal the beaker with plastic wrap and place it in a vacuum drying oven. 2 SO 4 10H 2 O fully melted. Take out the beaker, and use a ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com