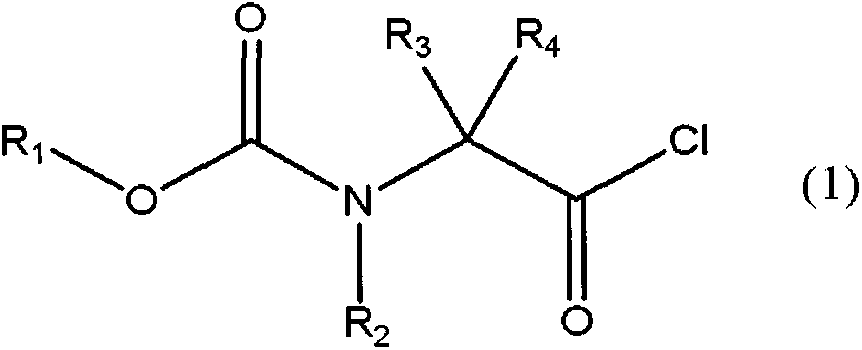
Method for producing amino acid amide derivative having fluorine-containing carbamate group, production intermediate thereof, and method for producing ethylene diamine derivative
A manufacturing method, the technology of alkyl chloroformate, which is applied to the preparation of carbamic acid derivatives, the preparation of organic compounds, chemical instruments and methods, etc., can solve the problems of long time, unfavorable economy, low yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
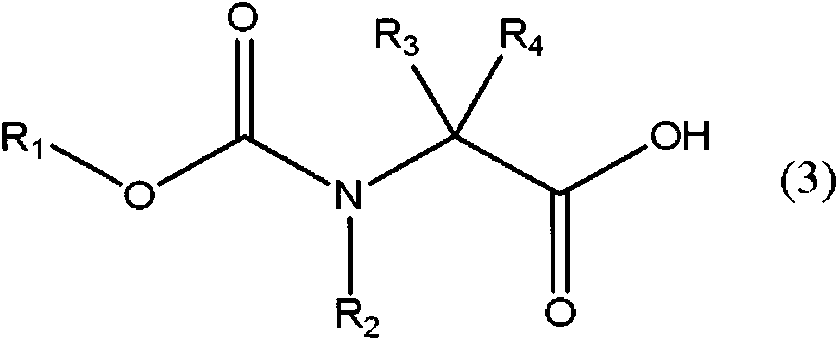
[0139] The production method of the compound represented by the general formula (3) is explained below.
[0140] The compound represented by the general formula (3) is obtained by reacting an amino acid with a fluorine-substituted alkyl chloroformate in the presence of water, as in Non-Patent Document 1. As the alkyl chloroformate substituted with fluorine, commercially available products or those synthesized by the method of Patent Document 2 or the like can be used.
[0141] As the preparation method of the compound represented by the general formula (3), by dissolving the amino acid in water, while keeping the pH of the reaction solution at 11 to 13, dropwise adding an alkyl chloroformate substituted by fluorine to allow the reaction to be carried out efficiently. to get.
[0142] As an example of the compound represented by general formula (3), the compound represented by general formula (3') can be used.
[0143]
[0144] (where, R 1 Represents an alkyl group with 1...
Embodiment 1
[0217](Example 1) Synthesis of N-(2,2,2-trifluoroethoxycarbonyl)-L-alanine
[0218]
[0219] 50.8 g of L-alanine and 100 g of water were charged into a 1000 mL four-necked flask equipped with a stirring device, cooled to 5° C., and adjusted to pH 12 with 32% by weight NaOH. While keeping the pH at 12±0.5 and below 10°C, add dropwise a mixed solution of 93.5 g of chloroformic acid 2,2,2-trifluoroethyl ester and 200 g of toluene, and then proceed for 1 hour while maintaining the pH at pH 12±0.5 Stir. Hydrochloric acid was added dropwise to adjust the pH to 1.5, and then the temperature was raised to 60° C. for liquid separation. The organic layer was concentrated under reduced pressure to obtain a white solid compound which was the title compound.
[0220] Yield 24.5g (yield 20%)
[0221] 1 H NMR (CDCl3) δ1.51 (3H, d, J = 7.32Hz), 4.40-4.53 (3H, m), 5.45 (1H, d, J = 8.79Hz).
[0222] LC-MS M+1(216)
Embodiment 2
[0223] (Example 2) Synthesis of N-(2,2,2-trifluoroethoxycarbonyl)-L-alanyl chloride (alaninochloride)
[0224]
[0225] In a 100mL four-necked flask with a stirring device, put 10g of dichloromethane, 1.0g of N-(2,2,2-trifluoroethoxycarbonyl)-L-alanine, N,N-dimethyl 1 drop of methyl formamide (hereinafter DMF), cooled to 5°C, 0.90 g of oxalyl chloride was added dropwise, and stirred for further 2 hours while maintaining the temperature at 5°C. After concentrating under reduced pressure, 10 g of dichloromethane was added to the obtained oily residue, stirred for 10 minutes, and then concentrated under reduced pressure to obtain an oily substance. The obtained compound as an oily substance was the title compound.
[0226] Yield 1.08g (yield 99.5%)
[0227] 1 H NMR (CDCl3) δ1.59 (3H, d, J=7.32Hz), 4.40-4.65 (3H, m), 5.48 (1H, br).
[0228] IR (ATR method) cm -1 3330, 1779, 1716, 1525, 1454, 1413, 1383, 1285, 1243, 1162, 1121, 1088, 1049, 985, 897, 839, 774, 739, 637, 554,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com