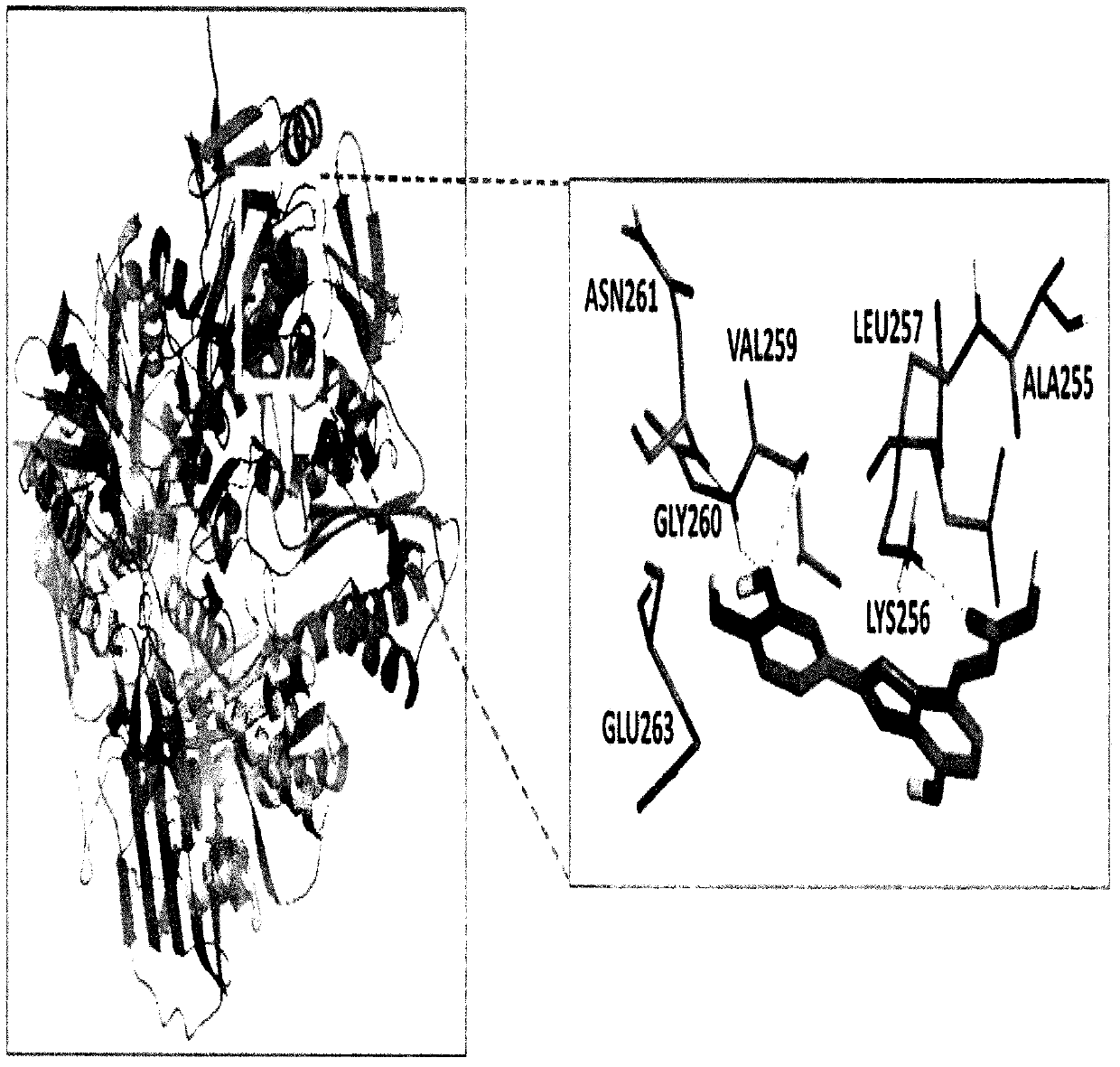
Use of 2-arylbenzofuran derivative in preparing of gout drugs
A kind of technology of benzofuran and derivatives, applied in the field of 2-arylbenzofuran derivatives and preparation thereof, and can solve problems such as related activities that have not yet been reported in literature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] 1. Synthesis of 2-arylbenzofuran derivatives 2
[0051] Tournefolic acid A (TAA) (2)
[0052] Accurately weigh Salvianolic acid C(1)(1equiv) in a 50mL two-neck round bottom bottle, add MeOH / H 2 04mL (5:1v / v), sonicate until the sample is completely dissolved, add an appropriate amount of inorganic base (NaOH or KOH) accurately weighed, and continue TLC under heating conditions (chloroform / methanol / formic acid 8:1:1v / v / v) The reaction was monitored until complete disappearance of starting material. After the reaction is complete, place it at room temperature, evaporate the solvent MeOH to dryness, add 15 mL of distilled water to dilute, and cool in an ice-water bath, add 10% HCl aqueous solution drop by drop and keep stirring until the pH is 3-4, then stop the dropwise addition. Add ethyl acetate (20mL×4) for extraction, combine the organic layers, wash with saturated NaCl solution, MgSO 4 Dry and concentrate under reduced pressure to obtain a crude product of the rea...
Embodiment 2
[0059]2. Synthesis of 2-arylbenzofuran derivatives 3
[0060] Methyl(E)-3-(2-(3,4-dihydroxyphenyl)-7-hydroxybenzofuran-4-yl)acrylate(3)
[0061] Take an appropriate amount of methanol and place it in an ice-water bath to cool for 15 minutes, and slowly add 2eq SOCl dropwise 2 , cooled the reaction for 30min, then added the compound Tournefolic acid A (2) (1eq), placed at room temperature, and TLC (chloroform / methanol / formic acid 8:1:1, v / v / v) continuously monitored the reaction until the raw material point was substantially disappear. The reaction product was concentrated under reduced pressure, and the crude product was isolated and purified by silica gel column chromatography (chloroform / methanol / formic acid 12:1:0.1, v / v / v) to obtain the target derivative 3 (yield 92%).
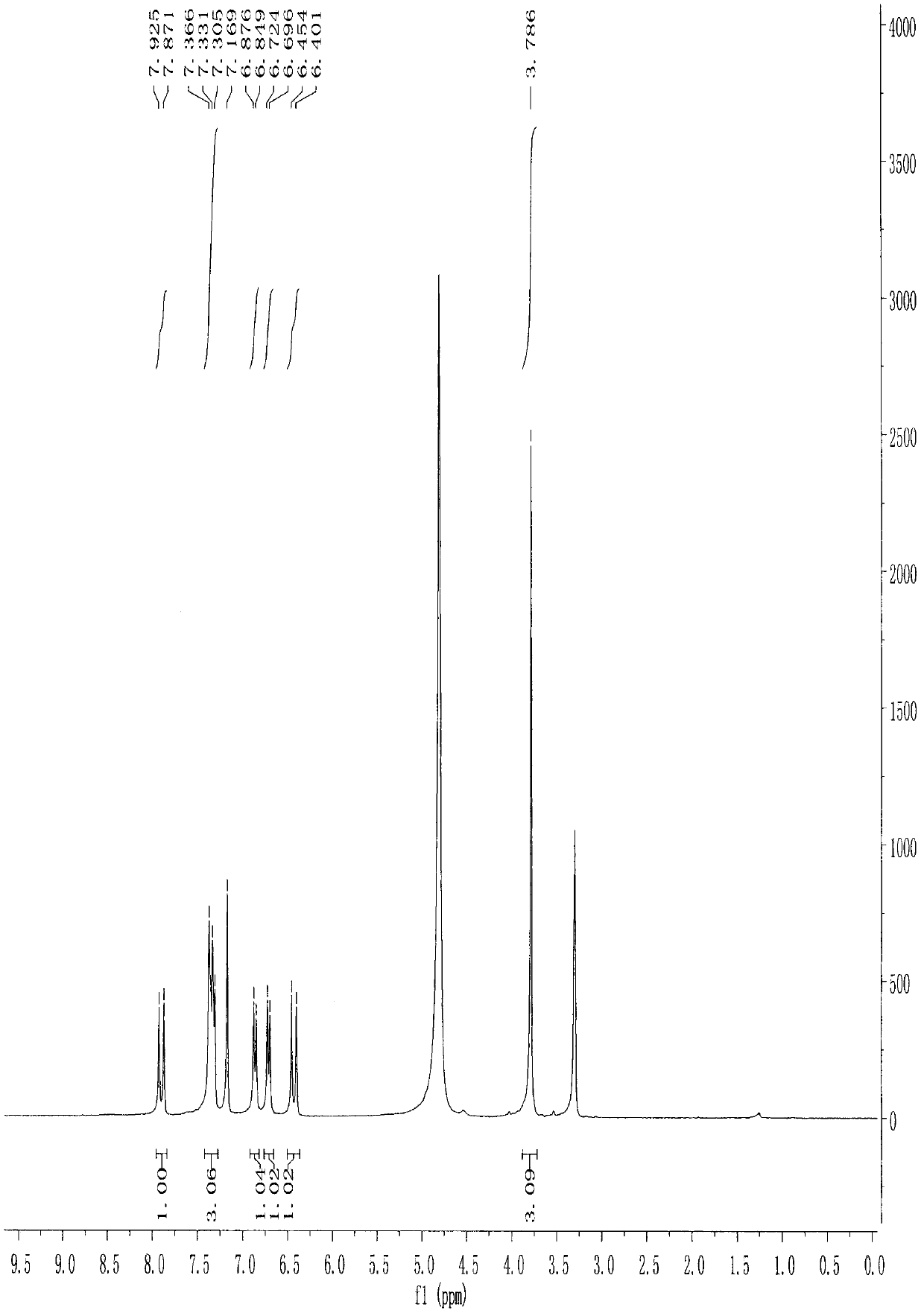
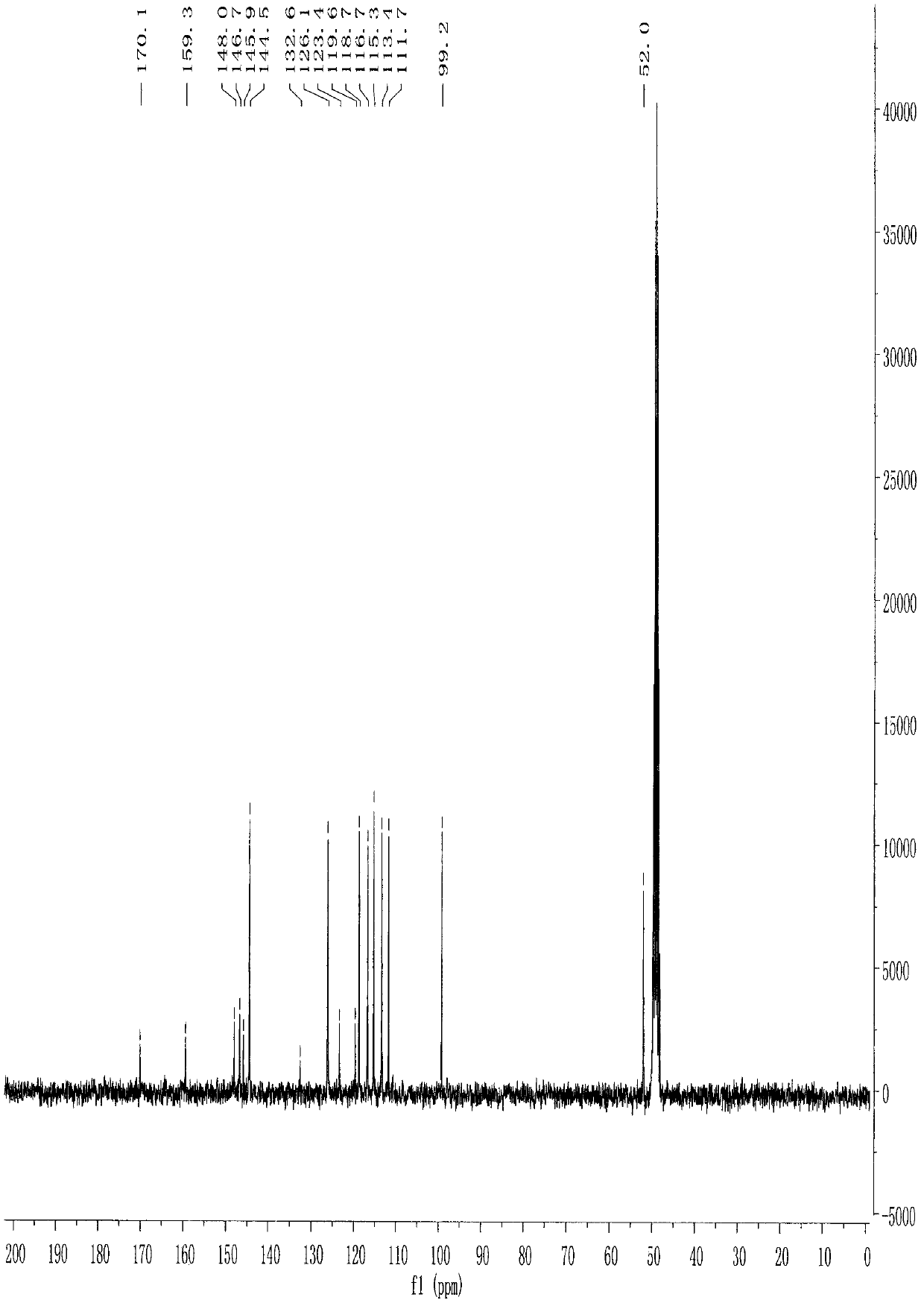
[0062] The structures of the reaction products obtained in the above steps were analyzed.
[0063] Physical and chemical properties: dark yellow powder (TLC R f =0.53 chloroform / methanol 4:1).
[0064...
Embodiment 3
[0068] 3. Synthesis of 2-arylbenzofuran derivatives 4
[0069] Ethyl(E)-3-(2-(3,4-dihydroxyphenyl)-7-hydroxybenzofuran-4-yl)acrylate(4)
[0070] Take an appropriate amount of ethanol and place it in an ice-water bath to cool for 15 minutes, and slowly add 2eq SOCl dropwise 2 , cooled the reaction for 30min, then added the compound Tournefolic acid A (2) (1eq), placed at room temperature, and TLC (chloroform / methanol / formic acid 8:1:1, v / v / v) continuously monitored the reaction until the raw material point was substantially disappear. The reaction product was concentrated under reduced pressure, and the crude product was separated and purified by silica gel column chromatography isocratic elution (chloroform / methanol / formic acid 12:1:0.1, v / v / v) to obtain the target derivative 4 (yield 81%).
[0071] The structures of the reaction products obtained in the above steps were analyzed.
[0072] Physical and chemical properties: dark yellow powder (TLC R f =0.57 chloroform / metha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com