Synthesis method for 4-sulfonamidophenylhydrazine hydrochloride
A technology of hydrazinobenzenesulfonamide and aminobenzenesulfonamide is applied in the field of synthesizing celecoxib intermediates to achieve the effects of inhibiting decomposition, accurately controlling reaction temperature and stably producing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
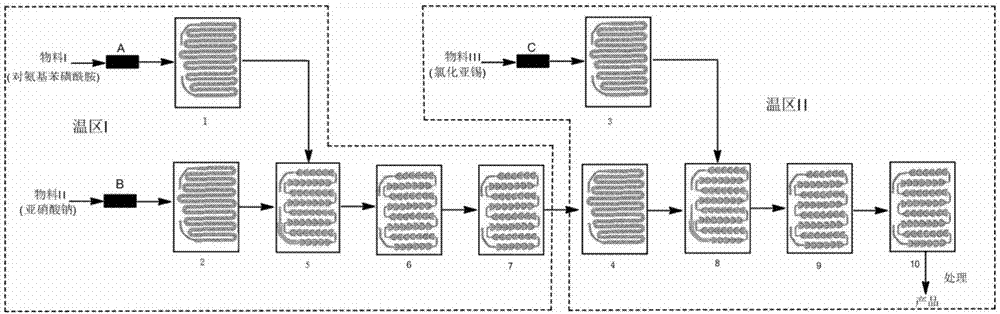
[0040]Weigh 80g of p-aminobenzenesulfonamide and add it to 800ml of water, then add 50ml of concentrated hydrochloric acid and stir to dissolve it as material I, weigh 32g of sodium nitrite and dissolve it in 500ml of water as material II, weigh 220g of stannous chloride and dissolve it with 800ml In the concentrated hydrochloric acid as material III, control metering pump A to make the flow rate of material I be 16ml / min, control metering pump B to make the flow rate of material II be 10ml / min, control metering pump C to make the flow rate of material III be 16ml / min, The mol ratio of sodium nitrite and p-aminobenzenesulfonamide is 1:1, the mol ratio of stannous chloride and p-sulfanilamide is 2.5:1, the residence time of diazotization reaction is 56 seconds, and the diazonium salt reduction The residence time of the reaction is 41 seconds, the reaction temperature of temperature zone I is 0°C, and the reaction temperature of temperature zone II is -10°C, collect the reaction ...
Embodiment 2
[0042] Weigh 100g of p-aminobenzenesulfonamide and add it to 800ml of water, then add 50ml of concentrated hydrochloric acid and stir to dissolve it as material I, weigh 40g of sodium nitrite and dissolve it in 500ml of water as material II, weigh 300g of stannous chloride and dissolve it with 1000ml In the concentrated hydrochloric acid as material III, control metering pump A to make the flow velocity of material I be 16ml / min, control metering pump B to make the flow velocity of material II be 9ml / min, control metering pump C to make the flow velocity of material III be 18ml / min, The mol ratio of sodium nitrite and sulfanilamide is 1:1, the mol ratio of stannous chloride and sulfanilamide is 3.0:1, the residence time of diazotization reaction is 58 seconds, and the diazonium salt reduction The residence time of the reaction is 34 seconds, the reaction temperature in temperature zone I is -5°C, and the reaction temperature in temperature zone II is -20°C, collect the reaction...
Embodiment 3
[0044] Weigh 80g of sulfanilamide and add it to 800ml of water, then add 40ml of concentrated hydrochloric acid and stir to dissolve it as material I, weigh 32g of sodium nitrite and dissolve it in 500ml of water as material II, weigh 220g of stannous chloride and dissolve it with 800ml In the concentrated hydrochloric acid as material III, control metering pump A to make the flow velocity of material I be 20ml / min, control metering pump B to make the flow velocity of material II be 12ml / min, control metering pump C to make the flow velocity of material III be 20ml / min, The mol ratio of sodium nitrite and p-aminobenzenesulfonamide is 1:1, the mol ratio of stannous chloride and p-sulfanilamide is 2.5:1, the residence time of diazotization reaction is 50 seconds, and the diazonium salt reduction The residence time of the reaction is 28 seconds, the reaction temperature of temperature zone I is 0°C, and the reaction temperature of temperature zone II is -5°C, collect the reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com