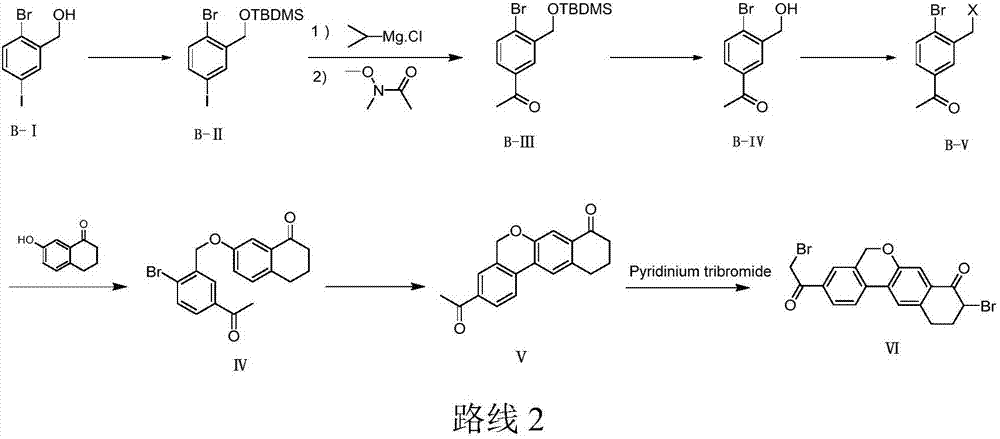
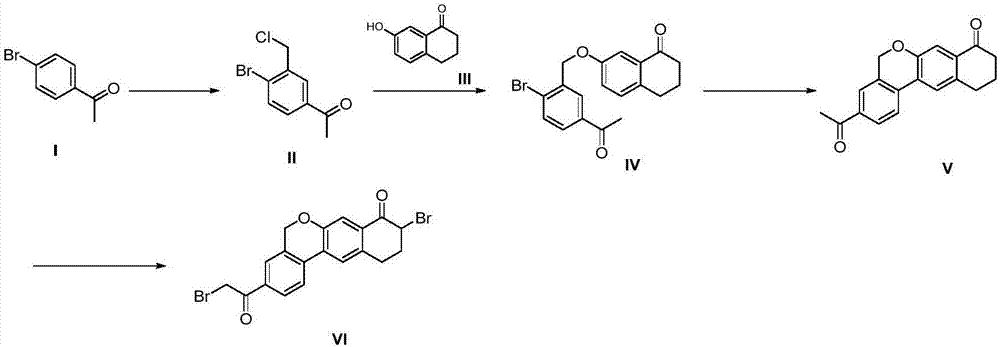
Preparation method of velpatasvir intermediate
A technology for velpatasvir and intermediates, which is applied in the field of preparation of velpatasvir intermediates, can solve the problems of unfavorable industrial production, low total yield, long route, etc., and achieve cheap and easy-to-obtain starting materials and excellent reaction conditions Gentle, high-yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Add the compound 4-bromoacetophenone (100g, 0.503mol) of formula I and 500ml 1,2-dichloroethane into a 1L three-necked flask, stir to dissolve, and add anhydrous aluminum chloride (254.8 g, 1.911mol), control the temperature not to exceed 45°C, cool down to 20-25°C after adding, slowly add paraformaldehyde (68g, 0.755mol) in batches, control the temperature to not exceed 45°C, after the addition is completed, rise to React at 45±2°C for 16-24h, monitor the reaction by HPLC, when the conversion rate of product II reaches 60-65%, lower the reaction temperature to ≤10°C, transfer to a 3L three-necked flask, add 1L of water dropwise to quench the reaction, Control the temperature to ≤35°C, stir and separate the layers, extract the water layer once with 200ml 1,2-dichloroethane, combine the organic phases, wash once with water, dry over anhydrous sodium sulfate, filter, concentrate, and use a mixed solvent of toluene and petroleum ether After beating, 62.2 g of off-white sol...
Embodiment 2
[0054] Add the compound 4-bromoacetophenone (100g, 0.503mol) and 500ml of dichloromethane into a 1L three-necked flask, stir to dissolve, and add anhydrous aluminum chloride (268.3g, 2.012mol) in batches under nitrogen protection. ), control the temperature not to exceed 40°C, and then lower the temperature to 20-25°C after adding, slowly add paraformaldehyde (90.6g, 1.006mol) in batches, control the temperature not to exceed 40°C, and rise to 40± React at 2°C for 16-24h, monitor the reaction by HPLC, when the conversion rate of product II reaches 60-65%, lower the reaction temperature to ≤10°C, transfer to a 3L three-necked flask, add 1L water dropwise to quench the reaction, control the temperature ≤ Stir and separate at 35°C, extract the water layer once more with 200m methylene chloride, combine the organic phases, wash once with water, dry over anhydrous sodium sulfate, filter, concentrate, and beat with a mixed solvent of toluene and petroleum ether to obtain 58.3g off-wh...
Embodiment 3
[0056] Add the compound 4-bromoacetophenone (100g, 0.503mol) and 500ml chloroform into a 1L three-necked flask, stir to dissolve, and add anhydrous aluminum chloride (201.3g, 1.509mol) in batches under nitrogen protection, Control the temperature not to exceed 50°C, and then lower the temperature to 20-25°C after adding, slowly add paraformaldehyde (45.4g, 0.503mol) in batches, control the temperature not to exceed 50°C, and rise to 50±2°C after the addition is complete React for 16-24 hours, monitor the reaction by HPLC, when the conversion rate of product II reaches 60-65%, lower the reaction temperature to ≤10°C, transfer to a 3L three-necked flask, add 1L of water dropwise to quench the reaction, and control the temperature to ≤35°C , stirred and separated, the aqueous layer was extracted once with 200ml chloroform, the organic phases were combined, washed once with water, dried over anhydrous sodium sulfate, filtered, concentrated, and beaten with a mixed solvent of toluen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com