Divinyl sulfamide linker as well as preparation and application thereof
A technology of bisethylene sulfonamide and ethylene sulfonamide, which is applied in the field of sulfur-sulfur bonds, can solve the problems of unsatisfactory stability of maleimide compounds and complicated preparation of monosulfone compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
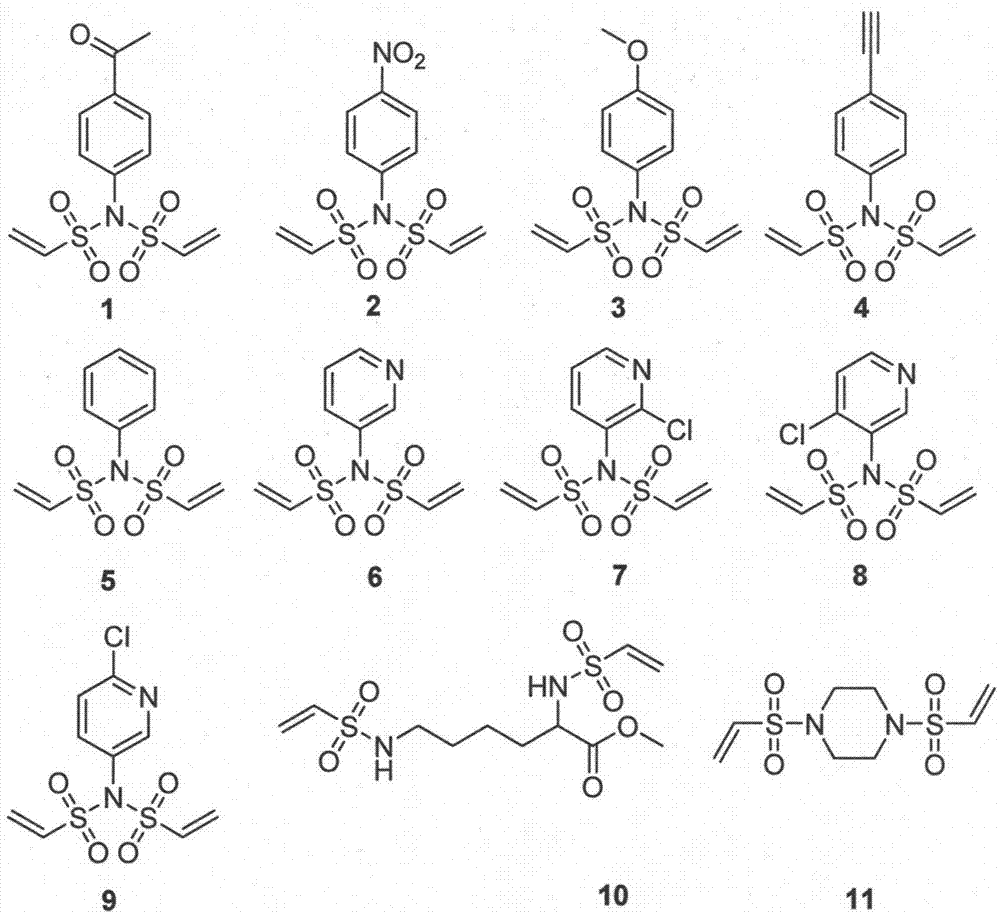
[0052] Preparation of bisethylenesulfonamide linker 1
[0053]
[0054] Using the general procedure A, add 675.8 mg of p-acetylaniline as the raw material, and add the others in equivalent amount, and react at 0°C for 10 minutes. Add 3ml CH in general step A 2 Cl 2 and 3g 60-100 mesh silica gel, mix well and spin dry. Ethyl acetate / petroleum ether = 1 / 6 was used as the eluent, and 770 mg of the product was obtained by passing through the column.
[0055] 1 H NMR (500MHz, CDCl 3 )δ8.01(d, J=8.5Hz, 2H), 7.38(d, J=8.5Hz, 2H), 7.07(dd, J=16.5, 9.9Hz, 2H), 6.30(d, J=16.6Hz, 2H), 6.18(d, J=9.8Hz, 2H), 2.62(s, 3H)ppm. 13 CNMR (126MHz, CDCl 3 )δ196.95, 138.48, 137.71, 136.07, 131.33, 130.30, 129.62, 26.94ppm. ESI-HRMS calcd for C 12 h 14 NO 5 S 2 [(M+H) + ]: 316.0313, found: 316.0296
Embodiment 2
[0057] Preparation of bisethylenesulfonamide linker 2
[0058]
[0059] Using the general procedure A, add 552.5 mg of p-nitroaniline as the raw material, and add the others in equivalent amounts, and react at 0°C for 1 hour. Add 3ml CH in general step A 2 Cl 2 and 2.5g 60-100 mesh silica gel, mix well and spin dry. Using ethyl acetate / petroleum ether=1 / 6 as eluent, the product was passed through the column to obtain 100 mg.
[0060] 1 H NMR (500MHz, CDCl 3 )δ8.33-8.24 (m, 2H), 7.49-7.42 (m, 2H), 7.07 (dd, J=16.5, 9.8Hz, 2H), 6.31 (dd, J=16.5, 0.9Hz, 2H), 6.22 (dd, J=9.9, 0.9Hz, 2H)ppm. 13 C NMR (126MHz, CDCl 3 )δ148.85, 139.24, 135.86, 132.19, 130.80, 124.90ppm. EI-GCMS calcd forC 10 h 11 N 2 o 6 S 2 (M): 317.9980, found: 218.20
Embodiment 3
[0062] Preparation of bisethylenesulfonamide linker 3
[0063]
[0064] Using the general procedure A, add 1.23 g of p-methoxyaniline as the raw material, and add the others in equivalent amount, and react at 0°C for 10 minutes. Add 10ml CH in general step A 2 Cl 2 and 5g 60-100 mesh silica gel, mix well and spin dry. Using ethyl acetate / petroleum ether = 1 / 6 as eluent, the product was passed through the column to obtain 1.93 g of the product.
[0065] 1 H NMR (500MHz, CDCl 3 )δ7.22-7.14(m, 2H), 7.04(dd, J=16.6, 9.9Hz, 2H), 6.94-6.88(m, 2H), 6.28(d, J=16.6Hz, 2H), 6.13(d , J=9.8Hz, 2H), 3.82(s, 3H)ppm. 13 C NMR (126MHz, CDCl 3 )δ161.17, 136.21, 132.19, 129.70, 125.97, 114.91, 55.67ppm.ESI-HRMScalcd for C 11 h 14 NO 5 S 2 [(M+H) + ]: 304.0313, found: 304.0298
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com