Cefotiam hydrochloride crystal compound and preparation method thereof
A technology for cefotiam hydrochloride and crystalline compound, which is applied in the field of cefotiam hydrochloride crystalline compound and its preparation, can solve the problems of solvent residue and the like, and achieves low organic solvent residue, good fluidity and low preparation cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
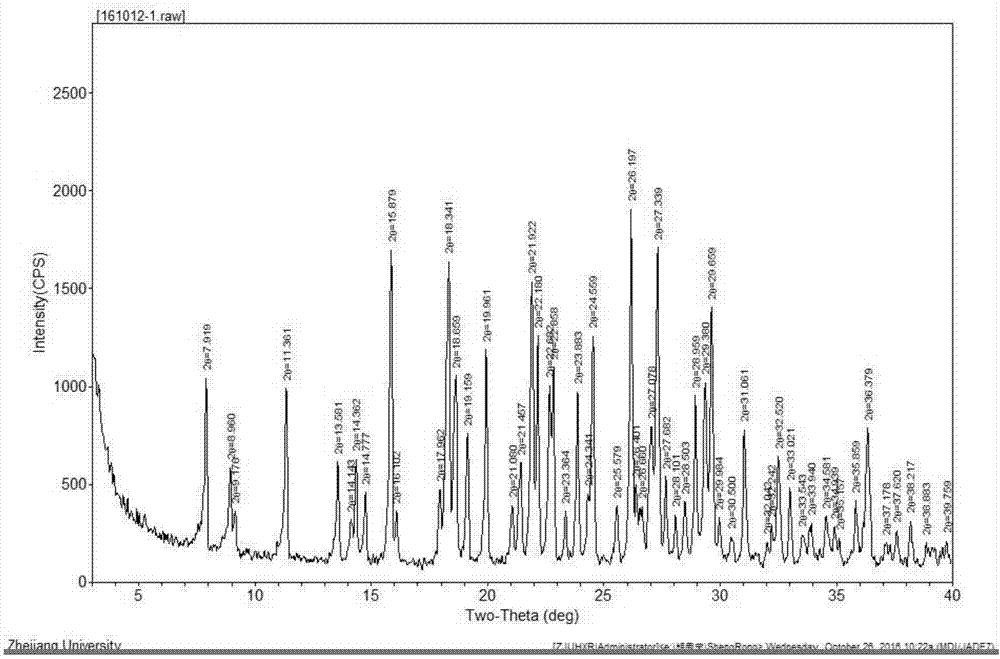
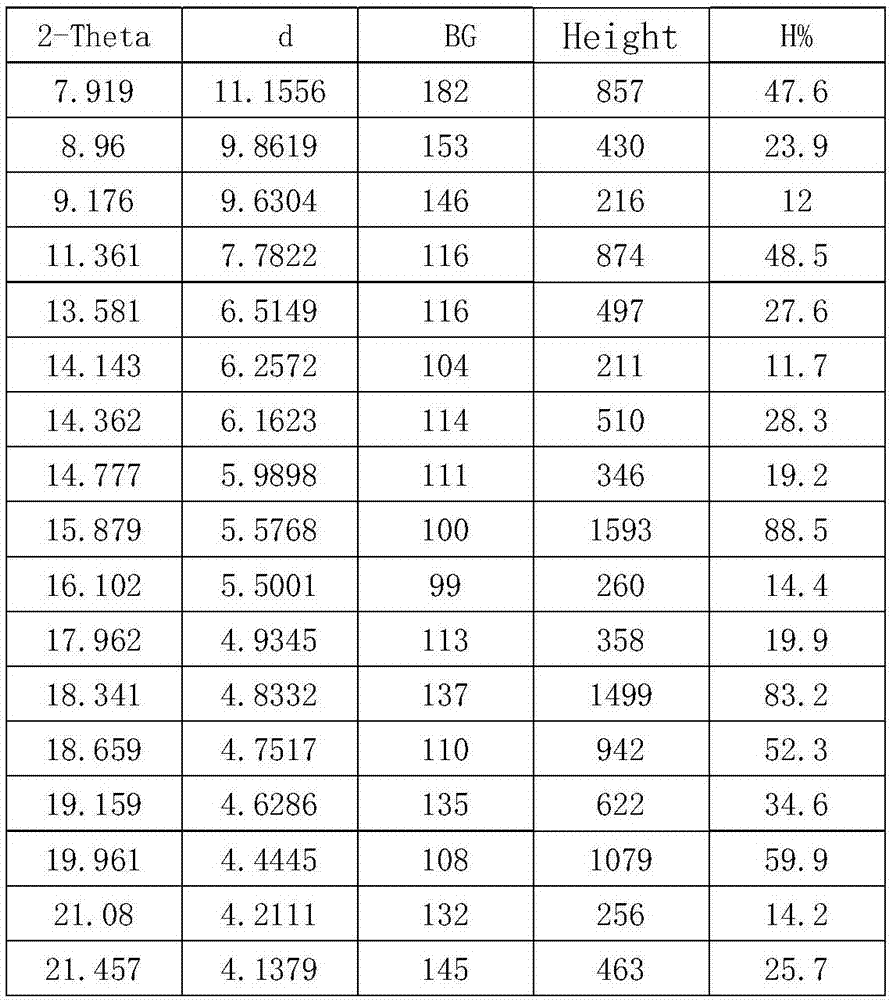
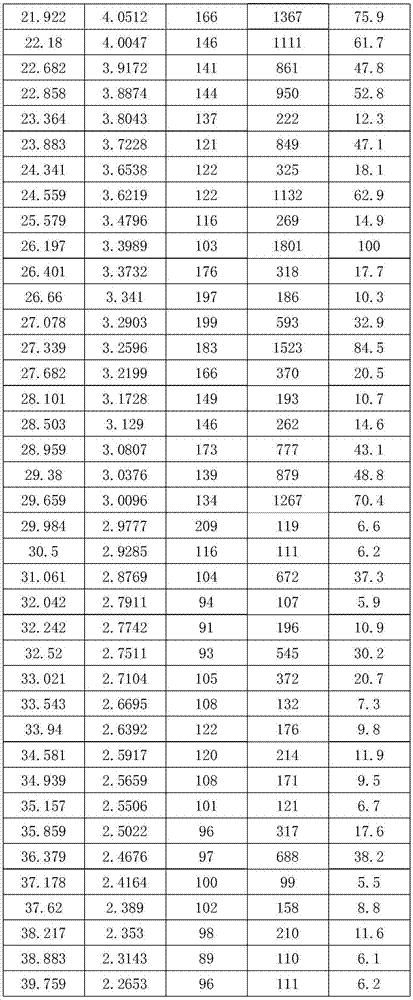
[0024] In a 5000mL flask, dissolve 562g (1mol) of crude cefotiam hydrochloride in 1200mL of methanol, add 150mL of concentrated hydrochloric acid and 600ml of ethyl acetate at 8°C, place the flask in an ultrasonic generator, and control the temperature at 23°C , turn on the ultrasonic seeding for 30 minutes, the ultrasonic frequency is 50KHz, and then stir for 30 minutes, then slowly add 600mL ethyl acetate to the flask, control the temperature at 8°C and crystallize for 2 hours, filter, wash the filter cake twice with 500mL ethyl acetate slurry , vacuum drying at 25° C. for 30 minutes, and then rising to 50° C. for 2 hours to obtain 555 g of cefotiam hydrochloride crystal form compound, with a yield of 98.8%.
Embodiment 2
[0026] In a 5000mL flask, dissolve 562g (1mol) of crude cefotiam hydrochloride in 1200mL of DMF, add 150mL of concentrated hydrochloric acid and 600ml of ethyl acetate at 8°C, place the flask in an ultrasonic generator, and control the temperature at 23°C. Turn on the ultrasonic seeding for 30 minutes, the ultrasonic frequency is 50KHz, and then stir for 30 minutes, then slowly add 600mL ethyl acetate to the flask, control the temperature at 8°C and crystallize for 2 hours, filter, wash the filter cake twice with 500mL ethyl acetate slurry, Vacuum drying at 25°C for 30 minutes, and then vacuum drying at 50°C for 2 hours to obtain 168.6 g of cefotiam hydrochloride crystal form compound with a yield of 30%.
Embodiment 3
[0028] In a 5000mL flask, dissolve 562g (1mol) of crude cefotiam hydrochloride in 1200mL of methanol, add 75mL of concentrated sulfuric acid and 600ml of ethyl acetate at 8°C, place the flask in an ultrasonic generator, and control the temperature at 23°C , turn on the ultrasonic seeding for 30 minutes, the ultrasonic frequency is 50KHz, and then stir for 30 minutes, then slowly add 600mL ethyl acetate to the flask, control the temperature at 8°C and crystallize for 2 hours, filter, wash the filter cake twice with 500mL ethyl acetate slurry , vacuum drying at 25°C for 30min, and then rising to 50°C for vacuum drying for 2h to obtain 281g of cefotiam hydrochloride crystal form compound with a yield of 50%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com