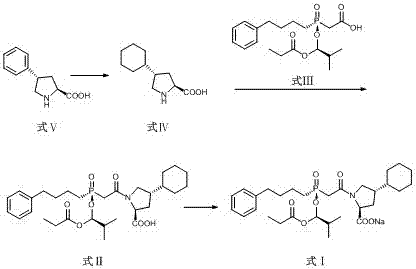
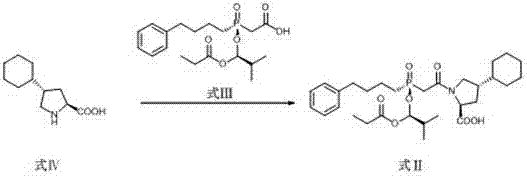
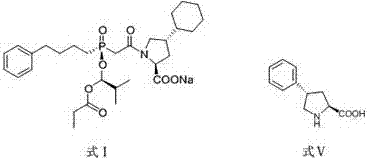
Preparation methods of antihypertensive Fosinopril sodium and key intermediate thereof
A technology for intermediates and compounds, applied in the field of preparation of trans-4-phenyl-L-proline (Formula V), which can solve the problems of low yield, high cost, difficulty in industrial production, and high ee value of products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0028] 1) Preparation of compound VII:
[0029]
[0030] Add methanesulfonic acid (3.24 g), triphenylphosphine (9.21 g) and toluene (70 ml) into the reaction flask, start stirring, the solution is a suspension, control the temperature of the suspension to 20°C, add azodicarboxylic acid Diisopropyl ester (7.95 g), keep the system temperature below 35°C. Add compound VIII (7.0 g) into the reaction flask, then add triethylamine (1.14 g), and stir for 10 min. Heating, heating up to 65°C, TLC spot plate tracking. After reacting for 3 hours, water (100 ml) and ethyl acetate (100 ml) were added to the reaction liquid, extracted twice, the organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, and distilled under reduced pressure to obtain 11.2 g of oil. Add the oil (11.2 g) and aqueous solution (25 ml) of sodium hydroxide (1.68 g) into a 250 ml reaction flask, stir vigorously at 5°C for 2 h, and adjust the pH to 6-7 with 6N hydrochloric a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com