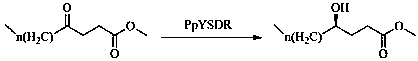Biological preparation method of chiral hydroxy acid ester
A hydroxy acid ester and biological preparation technology, applied in the direction of using vectors to introduce foreign genetic material, oxidoreductase, recombinant DNA technology, etc., can solve problems such as difficulty and pollution, and achieve high efficiency, improved chiral selectivity, The effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] A biological preparation method of a chiral hydroxy acid ester, comprising the following steps:
[0020] (1) The fatty acid is oxidized by ketolase globene monooxygenase to generate 4-oxo fatty acid;
[0021] (2) 4-oxo fatty acid was asymmetrically reduced by short-chain dehydrogenase PpYSDR-M85Q / L136A to obtain R-hydroxy acid ester.
[0022] Among them, the ketolase spheroidene monooxygenase is EC 1.14.15.9-spheroidene monooxygenase, which contains heme, participates in the biosynthesis of cyclin and 2,2'-dioxy-spiroflavin, and is derived from Rhodobactercapsulatus, Rhodobacter sphaeroides, Rhodovulum sulfidophilum, Rubrivivax gelatinosus.
[0023] Wherein the step 2 reaction is shown in the following formula:
[0024]
[0025] The short-chain dehydrogenase PpYSDR-M85Q / L136A is produced by mutation transformation of the wild-type short-chain dehydrogenase PpYSDR, which specifically includes the following process:
[0026] Mutant construction:
[0027] The oligon...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com