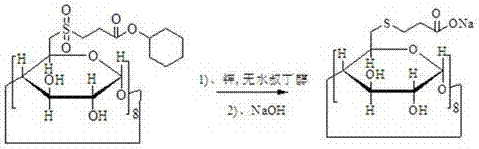Preparation method of sugammadex
A technology of sodium gluconate and sodium hydroxide, applied in the field of polysaccharide preparation, can solve the problems of difficult control of reaction conditions, difficult purification, low process yield and the like, and achieves convenient industrial production, high reaction efficiency and low cost. low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
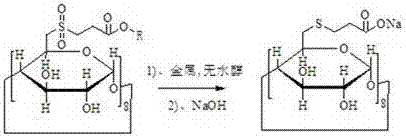
[0026] A preparation method of sugammadex sodium, using 6-deoxy-6-perhalogenated γ-cyclodextrin as a starting material, and preparing sugammadex sodium through the following steps in sequence
[0027] a. Substitution reaction of 6-deoxy-6-perhalogenated γ-cyclodextrin with 3-(chlorosulfonyl) propionate to obtain modified γ-cyclodextrin
[0028]
[0029] In the formula, R refers to 1-12 carbon chain alkanes, naphthenes and aromatic hydrocarbons;
[0030] 6-deoxy-6-perhalogenated γ-cyclodextrin is prepared by γ-cyclodextrin with triphenylphosphine and halogen;
[0031] b. After the γ-cyclodextrin modification and metal and anhydrous alcohol reduce the sulfone group to become a sulfide, then react with sodium hydroxide to obtain sugammadex sodium
[0032]
[0033] a Add tetrahydrofuran, N,N-dimethylformamide, N,N-dimethylacetamide and dimethyl sulfoxide to the reaction.
[0034] a base is added to the reaction.
[0035] The alkali is sodium hydroxide, potassium hydroxid...
Embodiment 1
[0039] Example 1: Preparation of 2-carbocarboxyethylsulfone-γ-cyclodextrin
[0040]
[0041] Dissolve 50g of dry 6-deoxy-6-perchloro-γ-cyclodextrin in dry 1L of N,N-dimethylformamide, add 22mL of triethylamine and cool down to -30°C, slowly drop After methyl 3-(chlorosulfonyl)propionate, control the temperature to 0°C and react for 2 hours, then slowly add the solution into the reaction liquid, then slowly add the reaction liquid into 1L of drinking water, a large amount of solids are precipitated, suction filtered, and 10 mL of cold water The filter cake was washed with N,N-dimethylformamide to obtain 55 g of off-white solid with a yield of 110%.
[0042] MS (m / z): 2393.35 [M+Na] + . 1 H-NMR (d6-DMSO): δ5.90-5.91 (16H, m), δ4.99-5.01 (8H, m), δ3.55-3.66 (32H, m), δ3.25-3.41 (16H, m), δ3.04-3.11 (8H, m), δ2.74-2.80 (24H, m), δ2.57-2.60 (16H, m).
Embodiment 2
[0043] Example 2: Preparation of 2-carboethoxyethylsulfone-γ-cyclodextrin
[0044]
[0045] Dissolve 25g of dry 6-deoxy-6-perbromo-γ-cyclodextrin in dry 500mL of N,N-dimethylacetamide, add 10mL of pyridine and cool down to -10°C, slowly add 3- After ethyl (chlorosulfonyl)propionate, control the temperature to 10°C and react for 1.5h, slowly add the solution into the reaction solution, slowly add the reaction solution into 500mL of drinking water, a large amount of solids are precipitated, suction filter, 10mL of cold The filter cake was washed with N,N-dimethylacetamide to obtain 28 g of an off-white solid with a yield of 112%.
[0046] MS(m / z): 2483.57[M+H] + . 1 H-NMR (d6-DMSO): δ5.91-5.93 (16H, m), δ4.99-5.04 (8H, m), δ3.76-4.03 (24H, m), δ3.24-3.43 (16H, m), δ3.09-3.13 (8H, m), δ2.75-2.83 (24H, m), δ2.56-2.60 (16H, m), δ1.27-1.33 (24H, m).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com