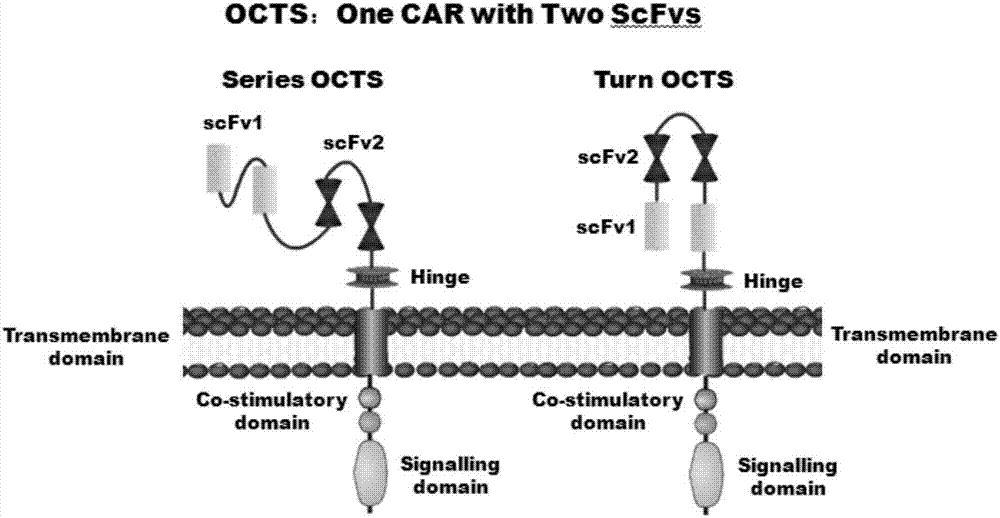
OCTS (One CAR (Chimeric Antigen Receptor) with two ScFvs (Single-chain variable Fragments)) technique based CAR-T (Chimeric Antigen Receptor-T cell immunotherapy) therapeutic vector for glioblastoma and construction method and application thereof
A malignant glioma, carrier technology, applied in the field of medical biology, can solve the problems that have not been overcome, and achieve the effects of improving curative effect, inhibiting immune escape, and ensuring reliable protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Example 1 OCTS-CAR-T cell construction
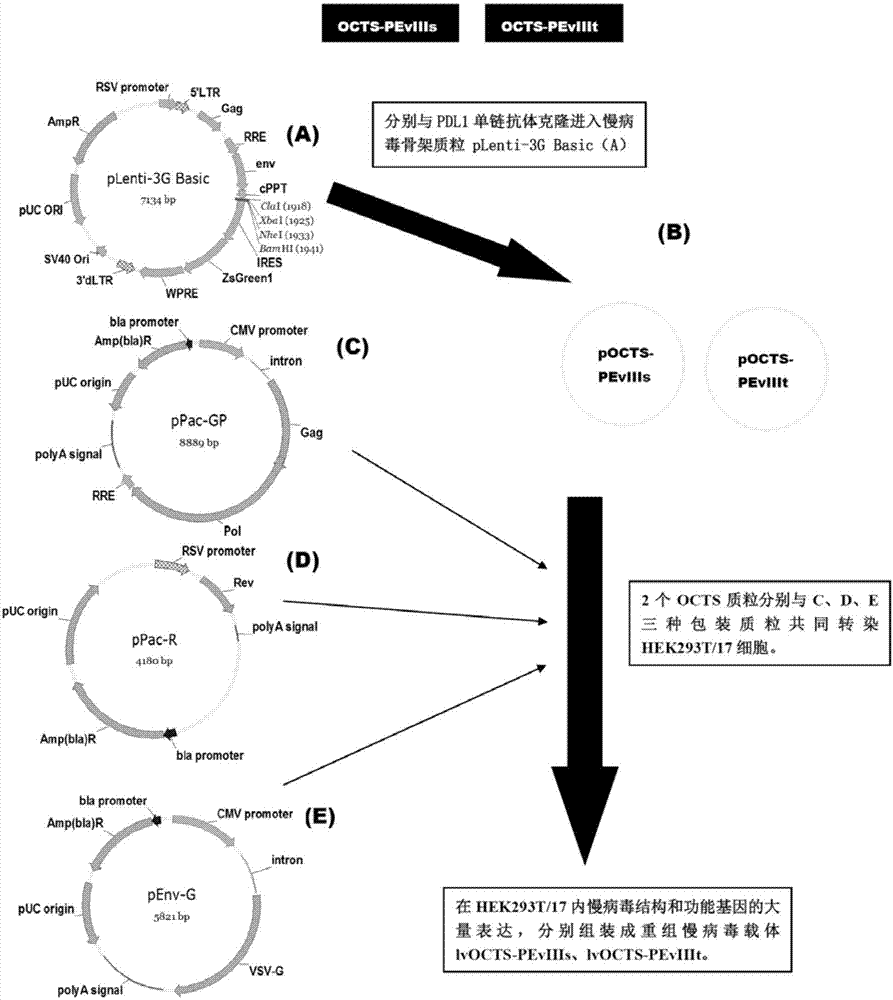
[0095] 1. Construction, purification and detection methods of recombinant lentiviral vector lvOCTS-PEvIIIs and lvOCTS-PEvIIIt.
[0096] See image 3 The method for constructing the recombinant lentiviral vector of the present invention is as follows:
[0097] 1. The human EF1α promoter (SEQ ID NO.14), OCTS structure [OCTS-PEvIIIs, OCTS-PEvIIIt] (CD8leader chimeric receptor signal peptide (SEQ ID NO.15), PDL1 single chain antibody light chain VL ( SEQ ID NO.16), PDL1 single chain antibody heavy chain VH (SEQ ID NO.17), EGFRvIII single chain antibody light chain VL (SEQ ID NO.18), EGFRvIII single chain antibody heavy chain VH (SEQ ID NO.19) ), antibody inner hinge Inner-Linker (SEQ ID NO.20), single-chain antibody hinge Inter-Linker (SEQ ID NO.21), CD8Hinge chimeric receptor hinge (SEQ ID NO.22), CD8Transmembrane chimeric receptor Somatic transmembrane region (SEQ ID NO.23), CD28 chimeric receptor costimulatory factor (SEQ ID NO.24), CD...
Embodiment 2
[0204] OCTS-CAR-T cell pathogen detection and expression detection.
[0205] 1. Endotoxin detection;
[0206] (1) The standard product of endotoxin is 15EU / piece;
[0207] (2) Limulus reagent sensitivity λ = 0.25EU / ml, 0.5ml / tube
[0208] (3). Dilution of endotoxin standard: take one endotoxin standard and dilute it with BET water to 4λ and 2λ respectively. Seal with parafilm and shake to dissolve for 15min; each dilution step should be mixed in a vortex during dilution. Mix on the device for 30s;
[0209] (4) Sample addition: Take several limulus reagents, add 0.5ml of BET water to dissolve each one, and distribute them into several endotoxin-free test tubes, each 0.1ml. Two of them are negative control tubes, add 0.1ml of BET water;
[0210] 2 positive control tubes, add 0.1ml endotoxin working standard solution with 2λ concentration;
[0211] 2 positive control tubes for samples, add 0.1ml sample solution containing 2λ endotoxin standard (1ml of the sample to be tested diluted 20 tim...
Embodiment 3
[0241] Example 3 Functional detection of OCTS-CAR-T cells.
[0242] 1. Evaluation of target cell killing effect.
[0243] (1) Culture target cells separately [PDL1 + K562, EGFRvIII + K562, PDL1 + EGFRvIII + K562, K562 cells] and effector cells [OCTS-CAR-T cells];
[0244] (2) Collect target cells 4x10 5 cells and OCTS-CAR-T cells 2.8x10 6 Cells, 800g, 6min centrifugation, discard the supernatant;
[0245] (3) Resuspend target cells and effector cells with 1ml D-PBS(-) solution, centrifuge at 800g for 6min, discard the supernatant;
[0246] (4) Repeat step 3 once;
[0247] (5) Resuspend the effector cells with 700ul medium (AIM-V medium+1-10% FBS), and resuspend the target cells with 2ml medium (AIM-V medium+1-10% FBS);
[0248] (6) Set the experimental wells with the ratio of effect to target of 1:1, 5:1, 10:1, and the grouping situation of effector cells co-incubated with single target cells and dual target cells are shown in Table 6, and set up a control group ( K562 cells), 3 replicat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com