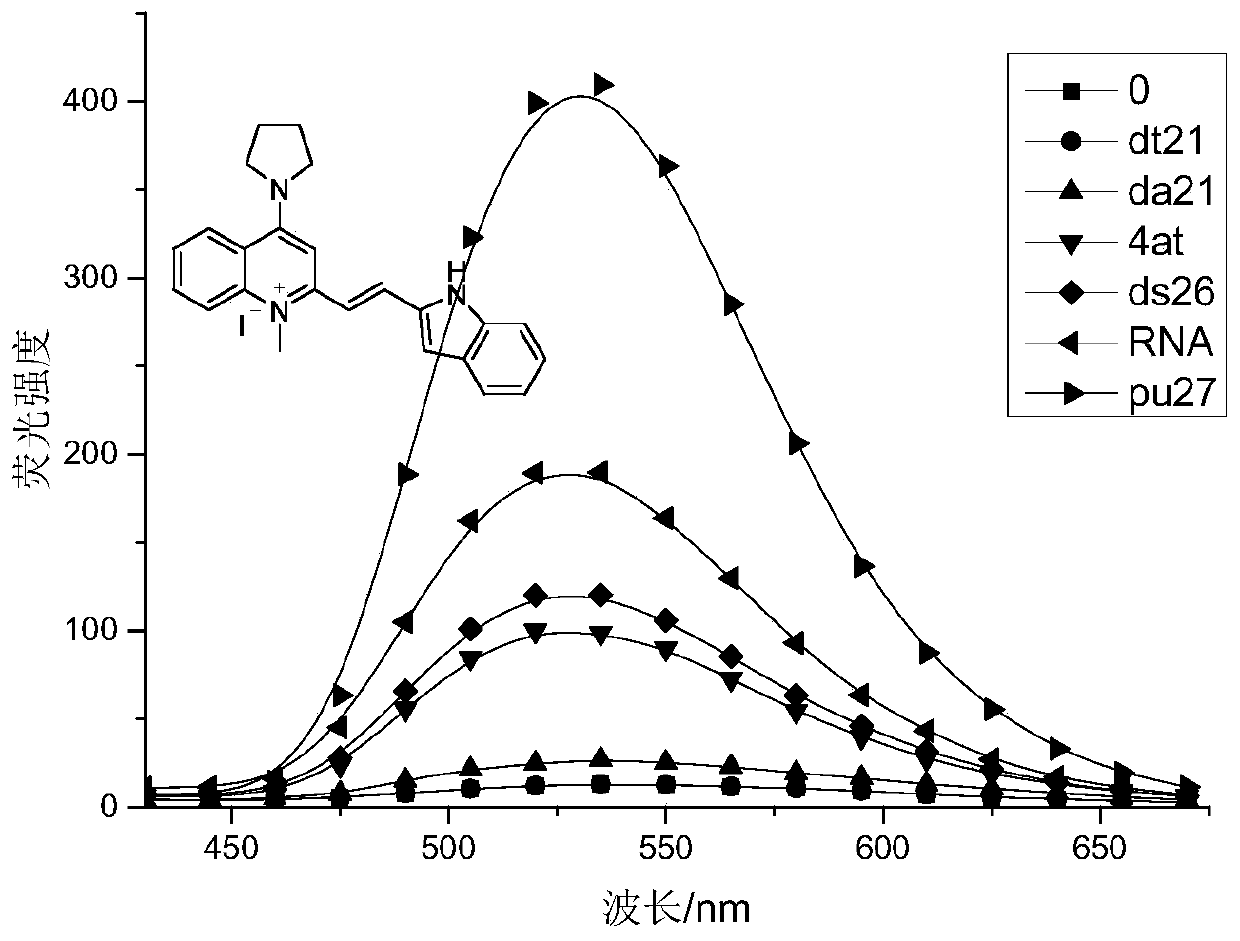
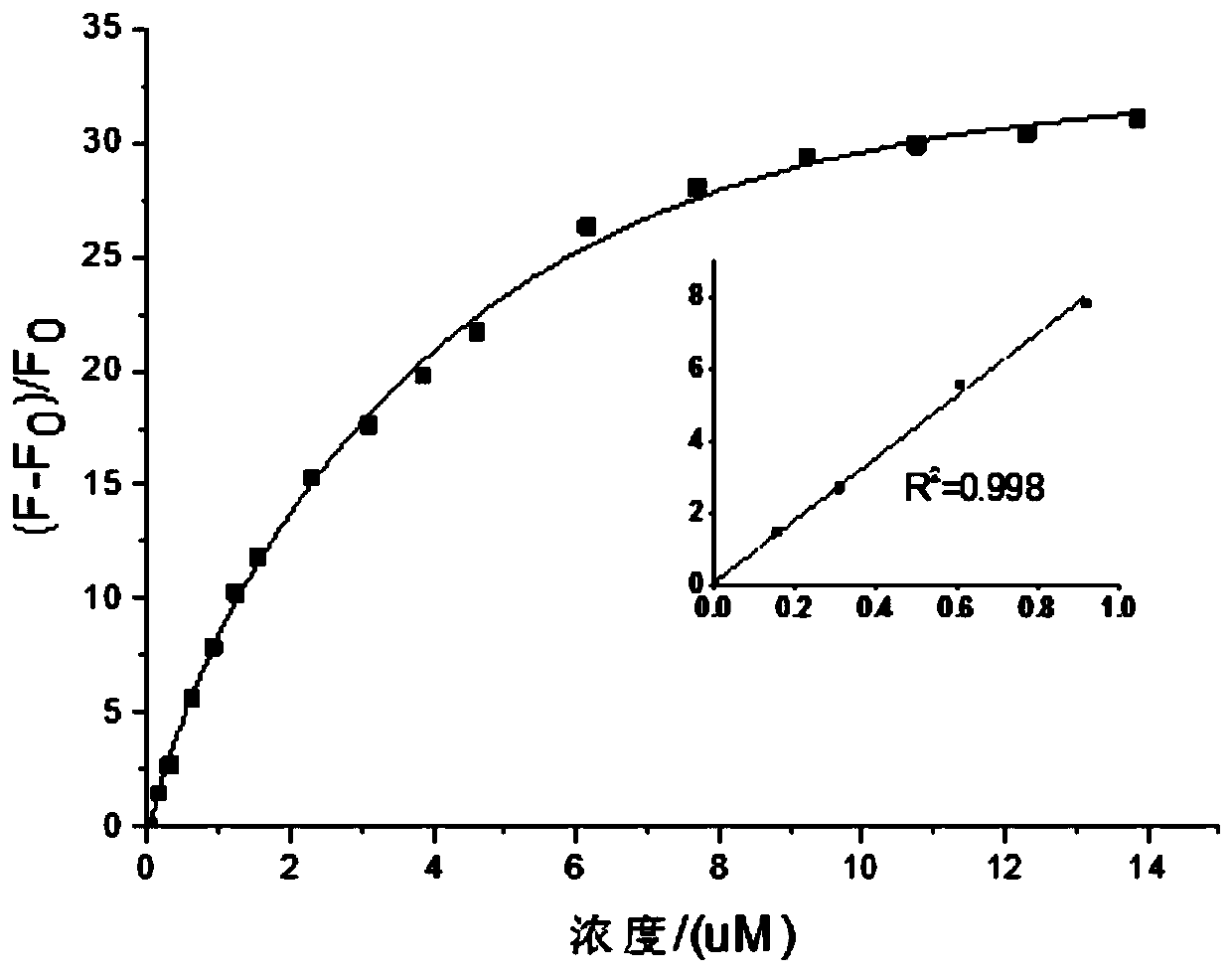
A kind of indole vinyl substituted quinoline derivatives and its preparation method and application
A technology of indolevinyl and quinolines, applied in the field of fluorescent probes, can solve the problems of inability to distinguish G-quadruplex structures, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] The present invention also provides a preparation method of indole vinyl substituted quinoline derivatives, comprising:
[0055] Formula (II) compound, formula (III) compound, R 2 -H reaction to obtain indole vinyl substituted quinoline derivatives;
[0056]
[0057] Among them, R 1 , R 3 independently selected from H, halogen, hydroxyl, C1-C6 alkoxy, C2-C15 imino, C1-C6 alkyl;
[0058] R 4 Alkyl group selected from C1~C6;
[0059] R 2 selected from piperidinyl, morpholinyl, pyrrolyl or NR 5 R 6 ;
[0060] R 5 , R 6 independently selected from H, -CH 3 、-CH 2 CH 3 、-CH 2 CH 2 OH, -CH 2 CH 2 CH 2 OH, formula (R-1), formula (R-2), formula (R-3), formula (R-4) or formula (R-5),
[0061] and R 5 , R 6 Not H at the same time.
[0062] According to the present invention, the present invention will formula (II) compound, formula (III) compound, R 2 -H reaction to obtain indolevinyl substituted quinoline derivatives; wherein, the limitation of each...
Embodiment 1
[0074] Embodiment 1: the synthesis of compound 2 (4-chloro-1,2-dimethylquinoline)
[0075] Weigh 0.2g (1.1236mmol) of 4-chloro-2-methylquinoline into a 25ml round bottom flask, add methyl iodide and solvent sulfolane, heat the mixture to 40-60°C, react for 18 hours, cool, add Shake it with anhydrous ether, filter it with suction, wash the solid several times, and weigh it after vacuum drying. Thin-layer chromatography shows that there is no by-product, and 0.345 g of pure product 2 is obtained with a yield of 95.8%.
[0076] 1 H NMR (400MHz, DMSO) δ8.56(d, J=8.4Hz, 1H), 8.46(d, J=8.3Hz, 1H), 8.22(t, J=8.1Hz, 1H), 8.01(t, J =7.9Hz, 1H), 7.55(s, J=7.4Hz, 1H), 4.20(s, 3H), 3.74(s, 1H), 2.68(s, 3H).
Embodiment 2
[0077] Embodiment 2: the synthesis of compound (I-a)
[0078] Weigh 0.0318g (0.001mol) of compound 2 and add it to a round-bottomed flask containing 10ml of ethanol, then add 0.217g, which is 1.5 times the molar amount of indole-2-carboxaldehyde, stir at room temperature for 5 minutes and then add 0.6ml of pyrrole was reacted at 80°C for 5 hours, cooled to room temperature, 10ml of ethyl acetate was added to the reacted solution, suction filtered after shaking, and the precipitate was washed with a small amount of ethanol, and compound (I-a) was obtained after vacuum drying. Brown solid 0.393g, its structure is as follows, the yield is 81.7%.
[0079] 1 H NMR (400MHz, DMSO) δ11.77(s, 1H), 8.51(d, J=8.4Hz, 1H), 8.16(d, J=8.8Hz, 1H), 8.00(t, J=7.8Hz, 1H ), 7.88(d, J=15.8Hz, 1H), 7.67(t, J=7.7Hz, 1H), 7.61(d, J=8.0Hz, 1H), 7.55(d, J=15.8Hz, 1H), 7.44(d, J=8.2Hz, 1H), 7.22(t, J=7.4Hz, 1H), 7.04(dd, J=9.0, 5.4Hz, 2H), 6.92(s, 1H), 4.12(s, 3H ), 3.98 (d, J=31.6Hz, 4H), 2.06 (s, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com