A kind of synthetic method of 2,3-dichloropyridine
A synthesis method and a technology for dichloropyridine, which are applied in the field of preparation of organic compounds, can solve the problems of expensive raw materials, many returned materials, and large amount of waste water, and achieve the effects of reducing production costs, avoiding cost input, and reducing process costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
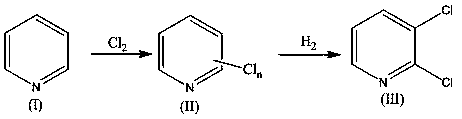
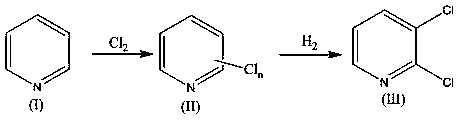
[0034] Mix 790 kilograms of pyridine, 3950 kilograms of water and 80 kilograms of iron powder, continuously feed 3000 kilograms of chlorine gas at 120 ~ 230 ° C, stir until the reaction is completed, stand and separate layers, and obtain 1500 kilograms of pyridinium chloride (II);
[0035] 1500 kg of pyridinium chloride (II) was mixed with 1500 kg of water, allowed to stand and separated to obtain 1440 kg of pyridinium chloride (II);
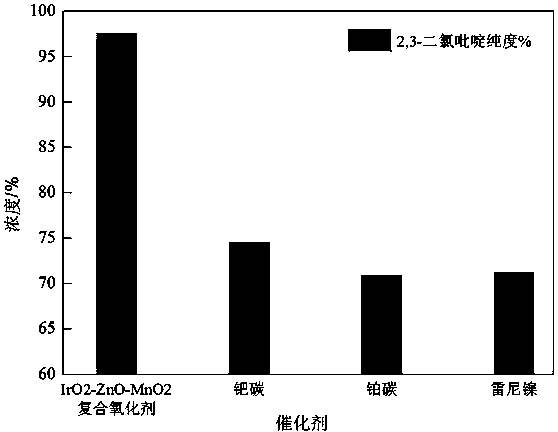
[0036] With 1440 kg of pyridinium chloride (II) and 5000 kg of water, 7.2 kg of nanometer IrO 2 -ZnO-MnO 2 Composite catalyst and 420 kg of pyridine are mixed, and the reaction is complete at 40~60°C and hydrogen pressure of 0.3~0.5MPa, and the nano-IrO is recovered by filtration. 2 -ZnO-MnO 2 Composite catalyst, the filtrate was separated by standing, and the oil layer was washed with 200 kg of water to obtain 1035.9 kg of 2,3-dichloropyridine with a purity of 98.3%;
[0037] 1035.9 kg of 2,3-dichloropyridine with a purity of 98.3% was purif...
Embodiment 2
[0045] Under the condition of 180~220℃ and ultraviolet light irradiation, the mixed steam of 790 kg of pyridine and 7900 kg of water is contacted with 3000 kg of chlorine gas to react; the mixed gas generated enters the reactor fixed with iron powder, and the chlorination product is collected , cooling, standing and stratifying to obtain 1560 kg of pyridinium chloride (II);
[0046] With 1560 kg of pyridinium chloride (II) and 7800 kg of water, 10 kg of nanometer IrO 2 -ZnO-MnO 2 Composite catalyst and 700 kg of sodium carbonate are mixed, under the conditions of 30~40℃ and hydrogen pressure of 0.3~0.5MPa, the reaction is complete, and the nano-IrO is recovered by filtration 2 -ZnO-MnO 2 Composite catalyst, the filtrate obtained 1050 kg of 2,3-dichloropyridine with a purity of 97.6% through standing and layering;
[0047] 1050 kg of 2,3-dichloropyridine with a purity of 97.6% was purified by rectification to obtain 903 kg of colorless and transparent 2,3-dichloropyridine wi...
Embodiment 3
[0055] Under the condition of 200~280℃ and ultraviolet light irradiation, the mixed steam of 790 kg of pyridine and 7900 kg of water is contacted with 3000 kg of chlorine gas to react; the mixed gas generated enters the reactor fixed with iron powder, and the chlorination product is collected , cooling, standing and stratifying to obtain 1430 kg of pyridinium chloride (II);
[0056] 1430 kg of pyridinium chloride (II) was mixed with 2860 kg of water, allowed to stand and separated to obtain 1380 kg of pyridinium chloride (II);
[0057] With 1380 kg of pyridinium chloride (II) and 6900 kg of water, 69 kg of nanometer IrO 2 -ZnO-MnO 2 Composite catalyst, 1380 kg of calcium carbonate mixed, under the condition of 60~80℃, hydrogen pressure of 0.8~1.0MPa, the reaction is complete, and the nano-IrO is recovered by filtration 2 -ZnO-MnO 2 Composite catalyst, the filtrate obtained 750 kilograms of 2,3-dichloropyridine with a purity of 93.3% through static stratification;
[0058] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com