Ketone compound and synthesis method thereof
A technology of ketone compounds and synthetic methods, which is applied in the field of organic synthesis, can solve the problems of poor substrate applicability, high toxicity of oxidants, unfavorable industrial production, etc., and achieve the effects of easy preparation and storage, less by-products, and high-efficiency methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1 synthesizes benzophenone
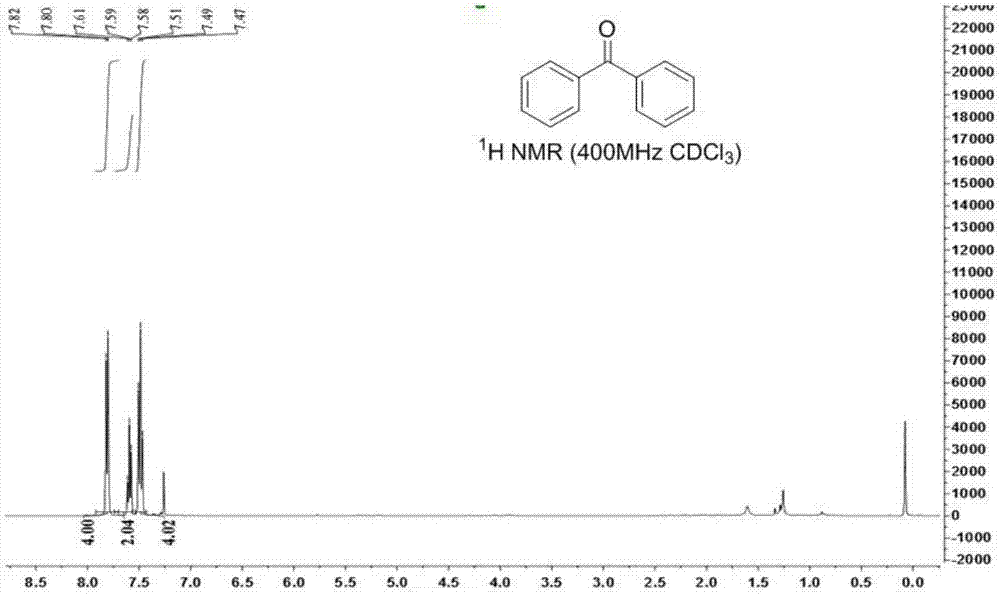
[0031] Weigh 0.1mmol tetraphenylethylene glycol, 0.2mmol 1-chloromethyl-4-fluoro-1,4-diazabicyclo[2.2.2]octane di(tetrafluoroborate) salt (SelectFlour) into 15mL A magnetic stirrer and 2mL N,N-dimethylformamide (DMF) were added to a pressure-resistant reaction tube, and the reaction was stirred at 80°C for 20h, as shown in formula (1). After the reaction, wash off the solvent with 10 mL of water, extract with 10 mL of ethyl acetate, dry with anhydrous sodium sulfate at 25°C for 15 minutes and filter, and finally use a rotary evaporator at -0.09MPa, 40°C to carry out vacuum distillation to remove organic solvent, the product benzophenone can be obtained, and the yield is 90%.
[0032] figure 1 is the NMR characterization collection of benzophenone, wherein, figure 1 for benzophenone 1 HNMR spectrum, figure 2 for benzophenone 13 C NMR spectrum. Benzophenone was confirmed by NMR characterization and the characteristic structu...
Embodiment 2
[0034] Example 2 Synthesis of 4,4'-dimethylbenzophenone
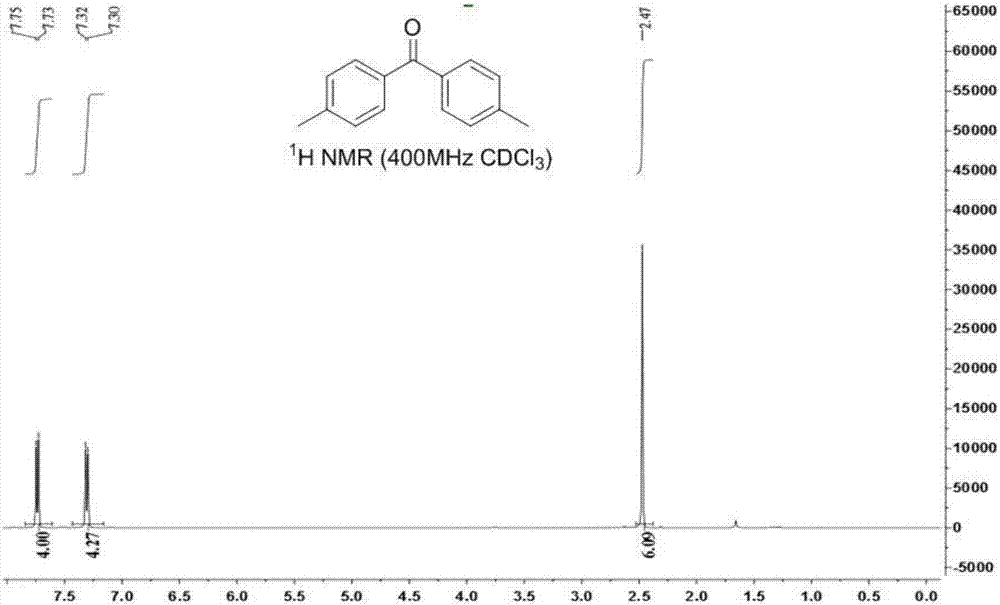
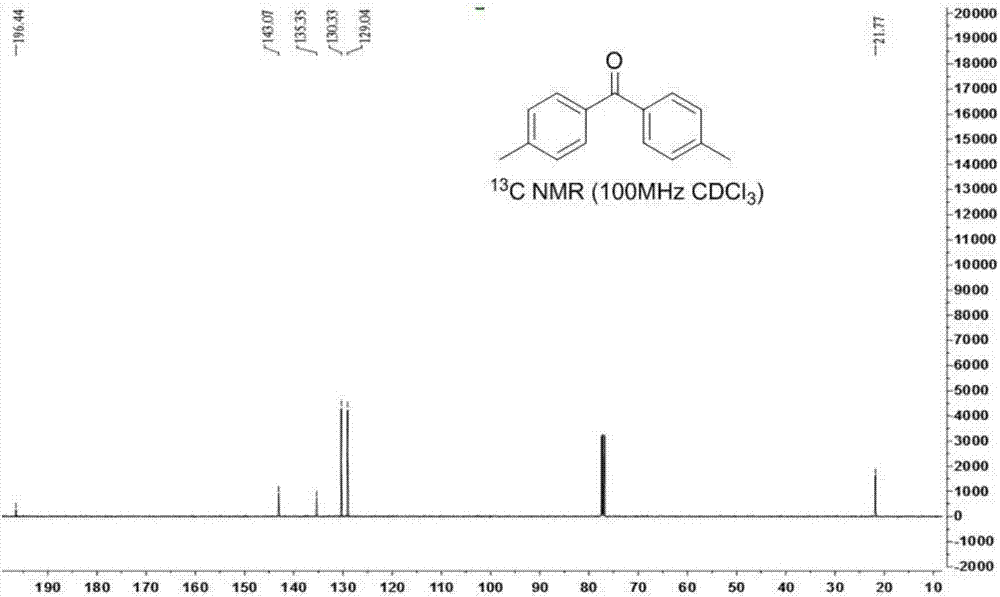
[0035] Weigh 0.1mmol 1,1,2,2-tetra-p-tolylethane-1,2-diol, 0.2mmol 1-chloromethyl-4-fluoro-1,4-diazabicyclo[2.2.2 ] Octane bis(tetrafluoroborate) salt was added to a 15mL pressure-resistant reaction tube, a magnetic stirrer and 2mL N,N-dimethylformamide were added, and the reaction was stirred at 80°C for 20h, as shown in formula (2). After the reaction, wash off the solvent with 10 mL of water, extract with 10 mL of ethyl acetate, dry with anhydrous sodium sulfate at 25°C for 15 minutes and filter, and finally use a rotary evaporator at -0.09MPa, 40°C to carry out vacuum distillation to remove organic solvent, the product 4,4'-dimethylbenzophenone can be obtained with a yield of 85%.
[0036] The NMR characterization spectrum of 4,4'-dimethylbenzophenone is as follows image 3 and Figure 4 shown. in, image 3 4,4'-Dimethylbenzophenone 1 HNMR spectrum, Figure 4 4,4'-Dimethylbenzophenone 13 C NMR spectrum. 4,4...
Embodiment 3
[0038] Example 3 Synthesis of 4,4'-dichlorobenzophenone
[0039]Weigh 0.1mmol 1,1,2,2-tetra-p-chlorophenylethane-1,2-diol, 0.2mmol 1-chloromethyl-4-fluoro-1,4-diazabicyclo[2.2. 2] Add octane bis(tetrafluoroborate) salt into a 15mL pressure-resistant reaction tube, add a magnetic stirrer and 2mL N,N-dimethylformamide, stir and react at 80°C for 20h, as shown in formula (3). After the reaction, the solvent was washed with 10 mL of water, extracted with 10 mL of ethyl acetate, dried with anhydrous sodium sulfate at 25°C for 10 minutes and filtered, and finally removed by vacuum distillation with a rotary evaporator at -0.095MPa and 35°C. organic solvent, the product 4,4'-dichlorobenzophenone can be obtained with a yield of 88%.
[0040] The NMR characterization spectrum of 4,4'-dichlorobenzophenone is as follows Figure 5 and Figure 6 shown. in, Figure 5 of 4,4'-dichlorobenzophenone 1 H NMR spectrum, Figure 6 of 4,4'-dichlorobenzophenone 13 C NMR spectrum. 4,4'-dichlo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com