Mesoporous Ag2O-MnO2 catalyst, preparation method thereof and application of catalyst
A catalyst and mesoporous technology, applied in the field of chemistry, can solve the problems of catalyst production and popularization restrictions and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] This example provides mesoporous Ag 2 O-MnO 2 Catalyst, it is prepared according to the following steps:
[0065] Step 1: Add 8.0g KIT-6 mesoporous silicon to 80ml, 0.91mol / L Mn(NO 3 ) 2 4H 2 O ethanol solution, after excessive impregnation and ultrasonic vibration, evaporate excess ethanol at 50°C, dry at 100°C, and roast at 200°C to obtain a mesoporous manganese oxide precursor; add the gained mesoporous manganese oxide precursor to 80ml, 0.91mol / L of Mn(NO 3 ) 2 4H 2 In O ethanol solution, after excessive impregnation and ultrasonic vibration, the excess ethanol was evaporated at 50°C, then dried at 100°C and calcined at 380°C to obtain a mesoporous manganese oxide precursor with a high filling degree. The mesoporous manganese oxide precursor was soaked in 2.0 mol / L NaOH solution, stirred by magnetic force for 60 min, and filtered to obtain a filter cake. The filter cake was soaked once more in the same NaOH solution, stirred by magnetic force for 60 min, and...
Embodiment 2
[0070] Step 1: Mesoporous MnO in this example 2 The preparation method and process parameters of the powder are the same as in Example 1.
[0071] Step 2: 1g of mesoporous MnO 2 Add the powder into the silver ammonia solution (0.016g of silver nitrate, 15ml of ammonia water with a mass fraction of 28%), and stir rapidly to form a mixed slurry. Then slowly add 15ml of hydrogen peroxide (mass concentration 30%) to make it react with the mixed slurry and release gas. After stirring for 120min, it is filtered 3 times, dried at 90°C, and roasted at 400°C to obtain mesoporous Ag. 2 O-MnO 2 catalyst.
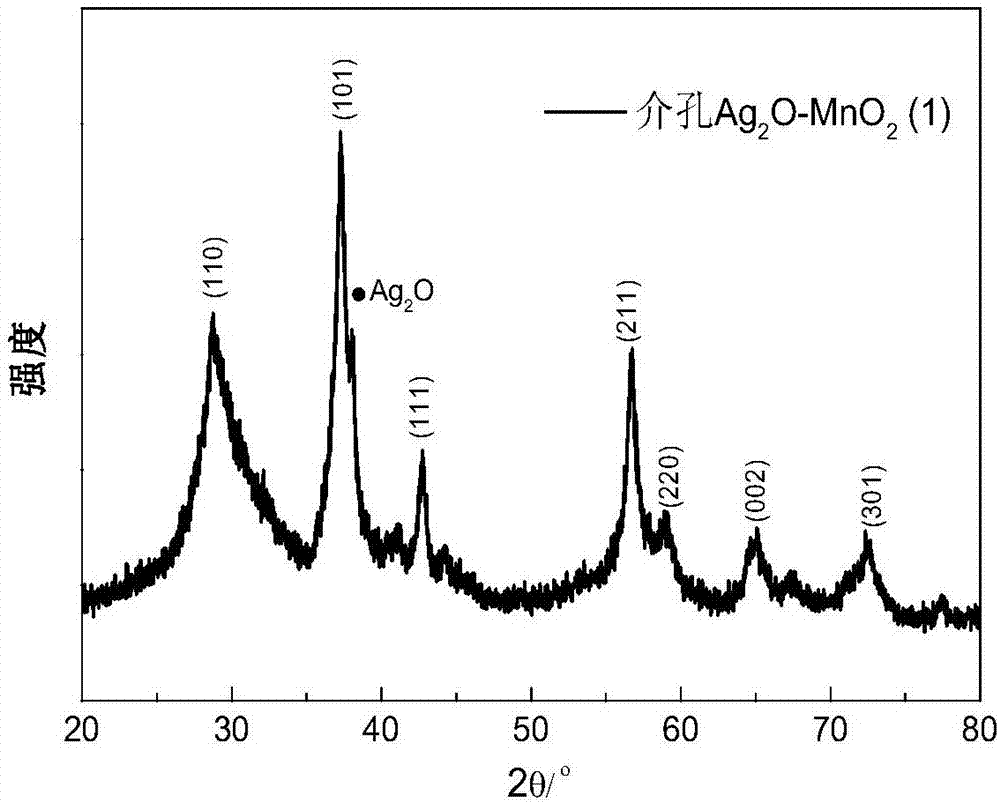
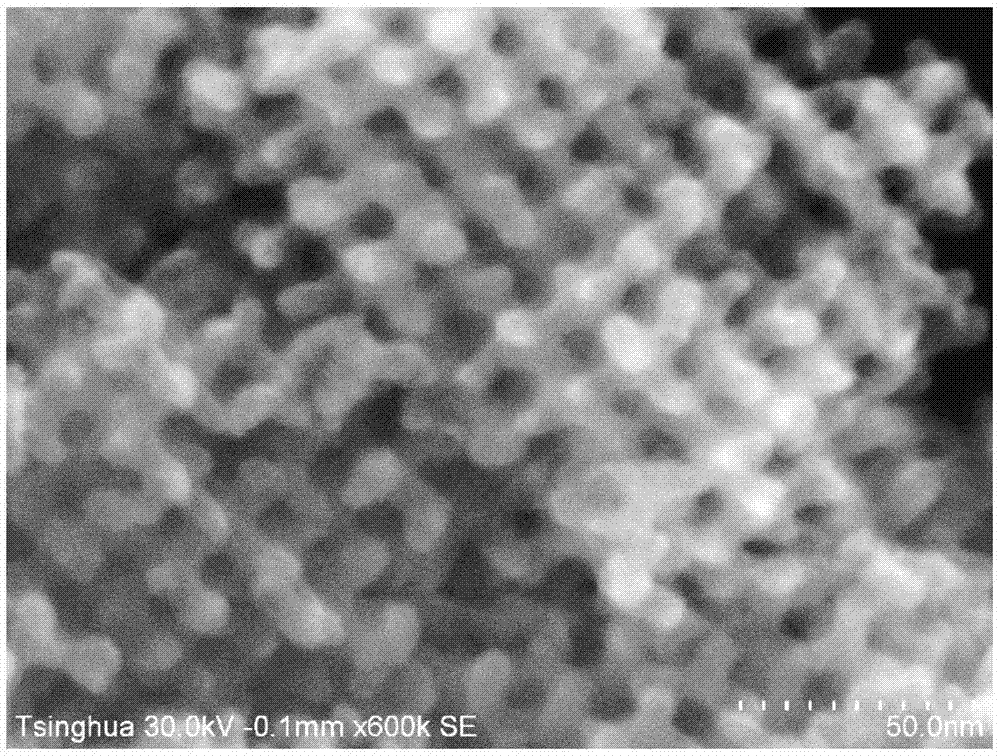
[0072] Using XRD to study the above mesoporous Ag 2 O-MnO 2 The powder was tested to characterize the presence of a silver oxide crystalline phase. It is beneficial for SEM to observe mesoporous Ag 2 O-MnO 2 surface structure features.
[0073] image 3 and Figure 4 Mesoporous Ag 2 O-MnO 2 XRD spectrum and SEM pattern of the catalyst. The XRD spectrum shows that there is...
Embodiment 3
[0075] Step 1: Mesoporous MnO in this example 2 The preparation method and process parameters of the powder are the same as in Example 1.
[0076] Step 2: 1g of mesoporous MnO 2Add the powder into the silver ammonia solution (0.008g of silver nitrate, 15ml of ammonia water with a mass fraction of 28%), and stir rapidly to form a mixed slurry. Then slowly add 15ml of hydrogen peroxide (mass concentration 30%) to make it react with the mixed slurry and release gas. After stirring for 120min, it is filtered 3 times, dried at 90°C, and roasted at 400°C to obtain mesoporous Ag. 2 O-MnO 2 catalyst.
[0077] Using XRD to study the above mesoporous Ag 2 O-MnO 2 The powder was tested to characterize the presence of a silver oxide crystalline phase. Figure 5 Mesoporous Ag 2 O-MnO 2 The XRD pattern of the catalyst. The spectrogram shows that the catalyst does not have silver oxide crystal phase, indicating that the Ag 2 O is evenly dispersed.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com