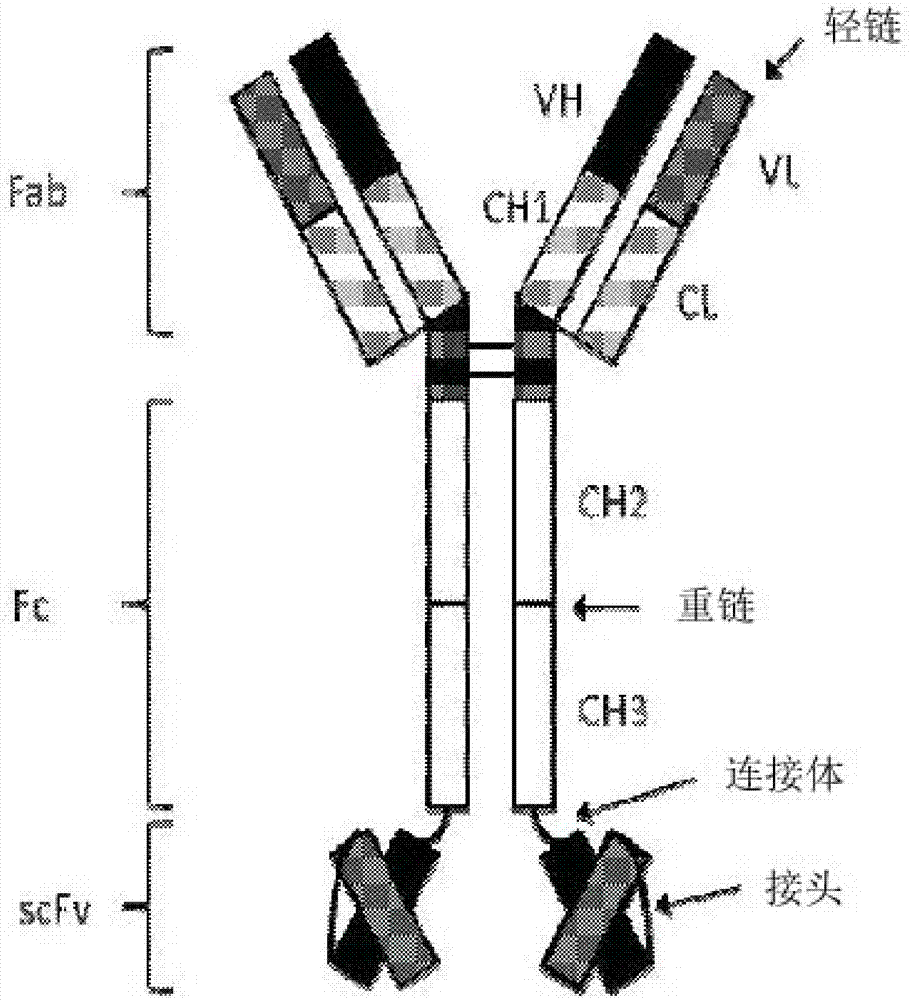
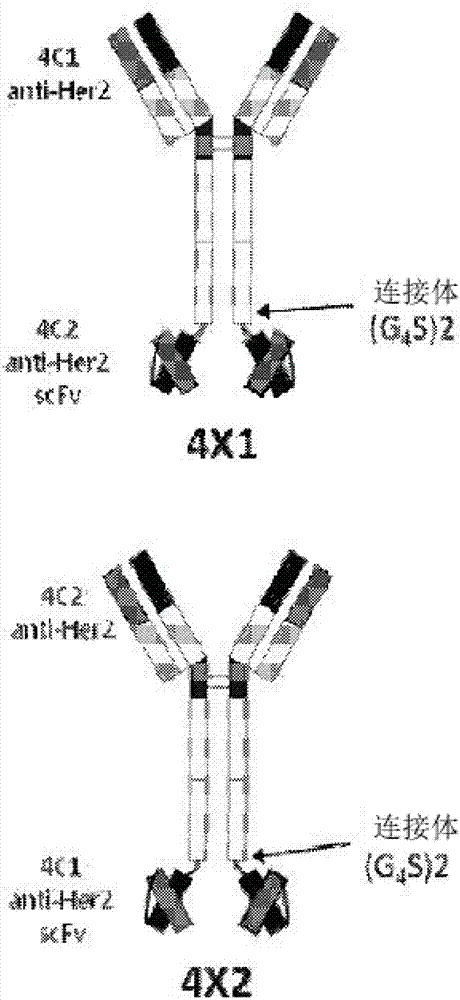
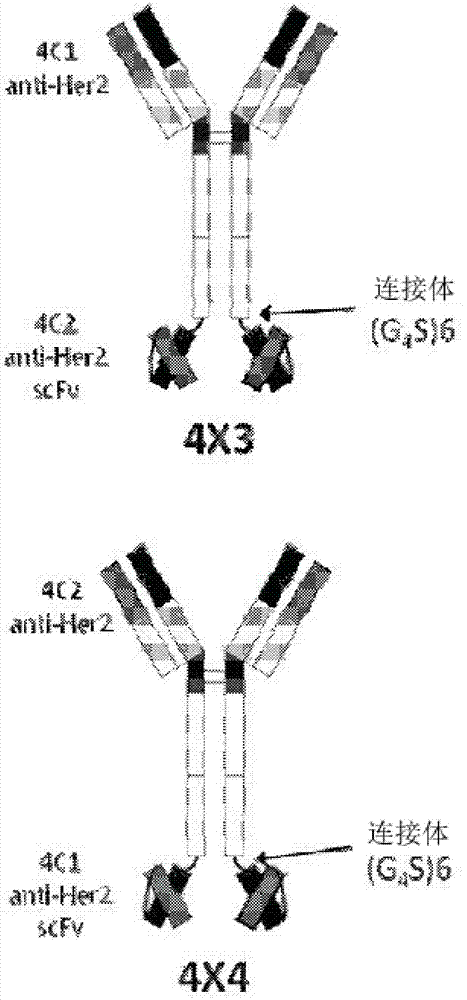
Bispecific tetravalent antibodies and methods of making and using thereof
A bispecific, binding specificity technology, applied in chemical instruments and methods, antibodies, antibody medical components, etc., can solve the problems of suboptimal and high cost of monoclonal antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0062] To assess the growth inhibitory potential of anti-HER2 antibodies, the effect on the proliferation of BT-474 cells (ATCC HTB-20, Manassas, Va.), a breast ductal carcinoma tumor cell line, was tested. Cells were seeded into 96-well tissue culture plates at a density of 6000 cells / well with 100 μl RPMI-1640 medium containing 1% fetal calf serum. After 4 hours, the test antibodies were added at different concentrations ranging from 0.0061 nM to 400 nM. Cells were cultured for 7 days in the presence of test antibodies. 20 μl of MTS reagent (Promega, Madison, Wl) was added to each well and the cells were incubated at 37°C for 2 hours. MTS is readily taken up by actively proliferating cells, reduced to formazan (which readily absorbs light at 490 nm), and then secreted into the culture medium. After incubation, OD490 values were measured using a BioTek (Winooski, VT) ELx800 absorbance reader. OD490 values for control cells (treated with medium only) were also obtained ...
example 2
[0065] The ability of anti-Her2 antibodies to be internalized by BT-474 cells was tested. A 1 mg aliquot of antibody standard PBS solution was reacted with Alexa Fluor 488 carboxylic acid, TFP ester (Thermo Fisher #A-10235, Waltham, MA) for 1 hour at room temperature. Unbound dye was removed by gel filtration using a Bio-Gel P-30 column. After conjugation, incubate an aliquot of 3x105 BT-474 cells with 50 nM of each Alexa488-labeled antibody in complete medium (RPMI-1640 + 10% FBS) at 37°C or 4°C (on ice) 1 hour. After incubation, cells were washed twice with ice-cold PBS in a cold centrifuge. Cells were then resuspended in 500 nM quenched rabbit-anti-Alexa488 antibody (Thermo Fisher #A-11094, Waltham, MA) or 500 nM rabbit IgG isotype control antibody (Jackson ImmunoResearch Laboratories #011-000-003, WestGrove, PA ) in either and incubated on ice for 30 minutes. Add two volumes of 2% paraformaldehyde to each sample and incubate for 10 min at room temperature. Cells were ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com