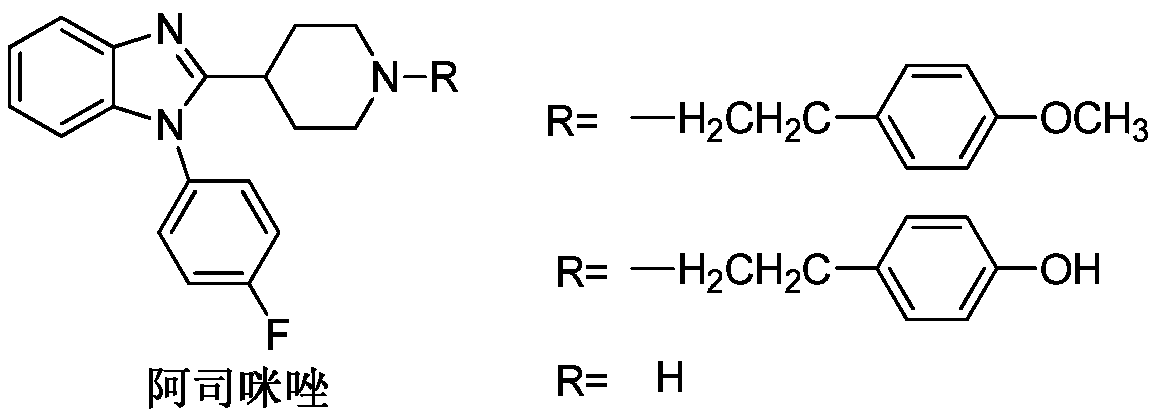A method for synthesizing key intermediates of astemizole and derivatives thereof
A technology of astemizole and intermediates, which is applied in the field of catalytic organic synthesis, can solve the problems that the catalyst cannot be recycled and reused, does not meet the requirements of atom economy, and does not meet the requirements of energy saving, and achieves green environmental protection, low cost and easy operation in the preparation process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
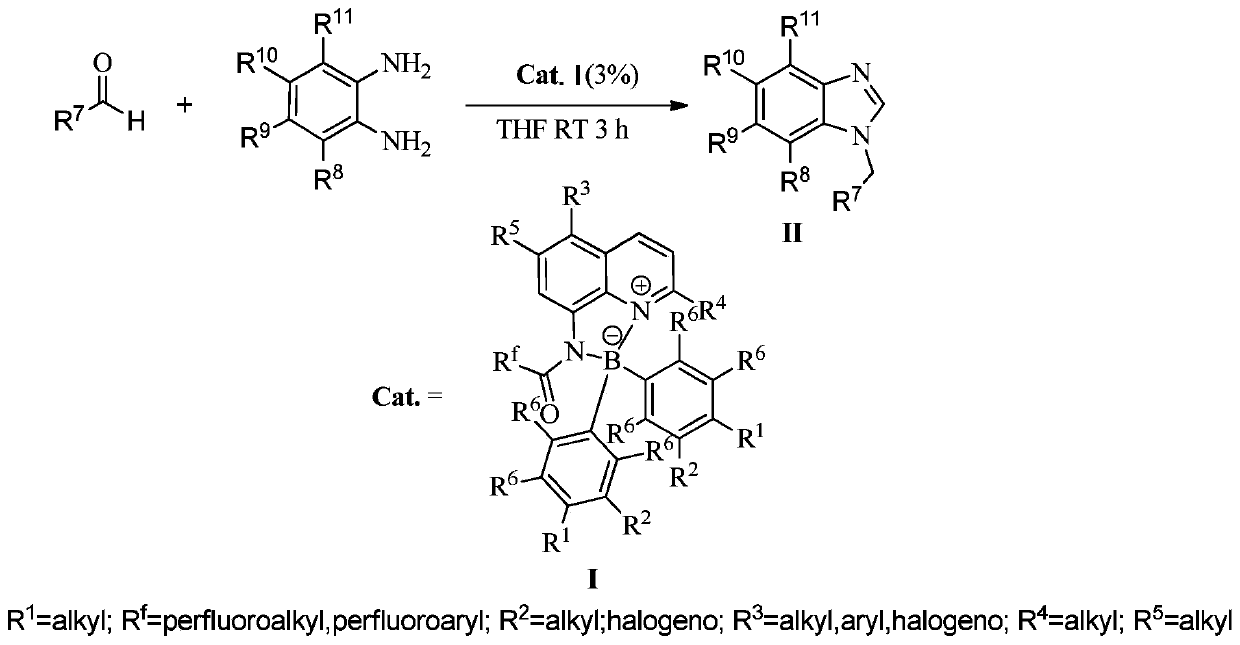
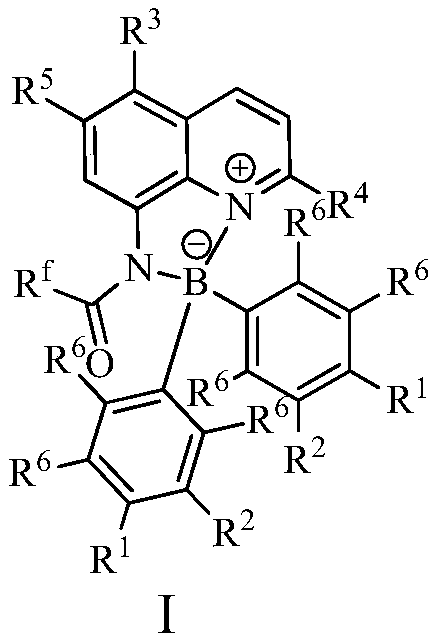
[0023] In a 100mL single-necked flask, add 0.01mol% Lewis acid-base bifunctional catalyst I (where R f = CF 3 ; 1 , R 2 , R 3 , R 4 , R 5 , R 6 =H), 0.2mol p-fluorobenzaldehyde (R 7 = 4-F-C 6 h 4 ), 0.1mol o-phenylenediamine (R 8 , R 9 , R 10 , R 11 =H), 15 mL of toluene, the reaction was stirred at 25° C. for 3 hours, followed by TLC until the reaction was complete. The reaction result is: product II (R 7 = 4-F-C 6 h 4 ; 8 , R 9 , R 10 , R 11 =H) The yield was 85%; after the catalyst system was reused 10 times, its catalytic performance did not decrease.
preparation example 2
[0025] In 100mL one-necked flask, add 0.03mol% Lewis acid-base bifunctional catalyst I (wherein R f = CF 3 ; 1 , R 2 , R 3 , R 4 , R 5 , R 6 =H), 0.2mol p-fluorobenzaldehyde (R 7 = 4-F-C 6 h 4 ), 0.1mol o-phenylenediamine (R 8 , R 9 , R 10 , R 11 =H), 15 mL of toluene, the reaction was stirred at 25° C. for 3 hours, followed by TLC until the reaction was complete. The reaction result is: product II (R 7 = 4-F-C 6 h 4 ; 8 , R 9 , R 10 , R 11 =H) The yield was 90%; after the catalyst system was reused 10 times, its catalytic performance did not decrease.
preparation example 3
[0027] In 100mL one-necked flask, add 0.08mol% Lewis acid-base bifunctional catalyst I (where R f = CF 3 ; 1 , R 2 , R 3 , R 4 , R 5 , R 6 =H), 0.2mol p-fluorobenzaldehyde (R 7 = 4-F-C 6 h 4 ), 0.1mol o-phenylenediamine (R 8 , R 9 , R 10 , R 11 =H), 15 mL of toluene, the reaction was stirred at 25° C. for 3 hours, followed by TLC until the reaction was complete. The reaction result is: product II (R 7 = 4-F-C 6 h 4 ; 8 , R 9 , R 10 , R 11 =H) The yield was 91%; after the catalyst system was reused 10 times, its catalytic performance did not decrease.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com