Transition metal phosphide serving as hydrogen preparation catalyst of hydrolysis reaction of borohydride
A borohydride and transition metal technology, applied in the field of transition metal phosphides as borohydride hydrolysis reaction hydrogen production catalysts, to achieve excellent catalytic efficiency, cycle and thermal stability, broad application prospects, and efficient hydrolysis hydrogen production effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
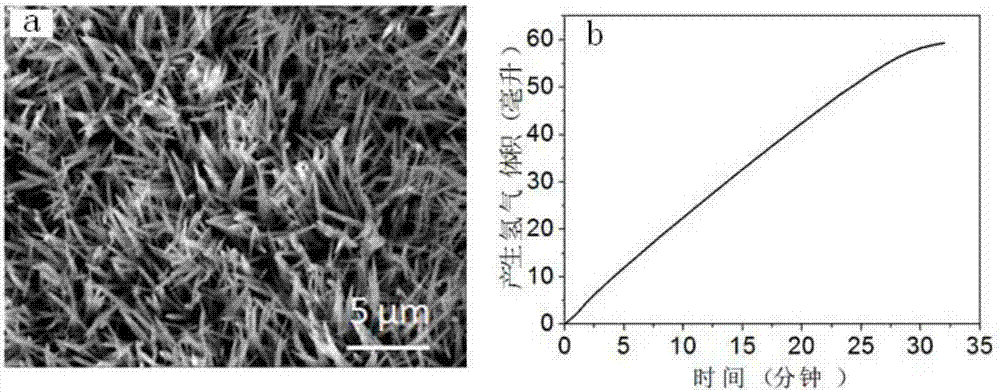
example 1
[0041] Step 1: Add 20mL of distilled water to the polytetrafluoroethylene lining, control the addition of 0.0146g of hexahydrate and cobalt nitrate, 0.00046g of ammonium fluoride, and 0.015g of urea per milliliter of water, that is, add 0.291g of hexahydrate and cobalt nitrate, 0.093 g ammonium fluoride and 0.30 g urea and stirred until the solids were completely dissolved to form a clear solution.
[0042] Step 2: Put the catalyst substrate titanium mesh into the reaction kettle lining of step 1, and seal the polytetrafluoroethylene lining into a stainless steel mold, place it in a constant temperature drying oven under closed conditions and heat it at 120°C for reaction 6h.
[0043] Step 3: After the reaction is completed, cool down to room temperature with the furnace, then take out the titanium mesh, wash it with distilled water and absolute ethanol in sequence, and place the washed titanium mesh in a vacuum drying oven and dry it in vacuum at 40°C for 24 hours , to obtai...
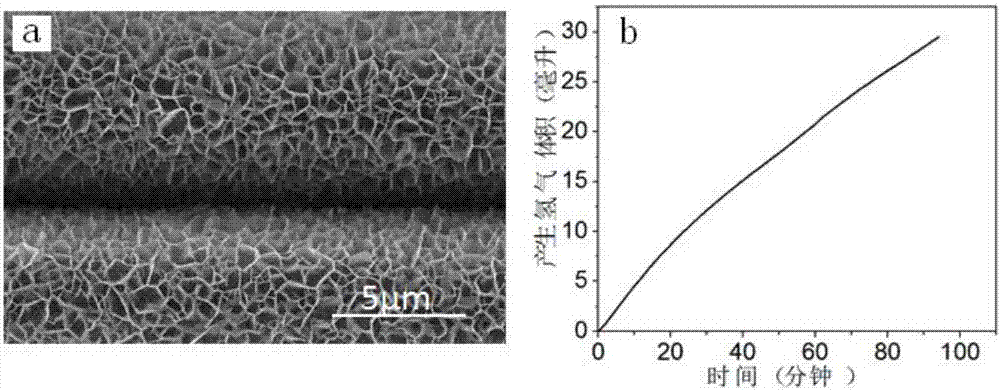
example 2
[0047] Step 1: Add 36mL of distilled water to the PTFE lining, that is, add 1.45395g of nickel nitrate and 1.4019g of hexamethylenetetramine, and stir until the solids are completely dissolved to form a transparent solution.
[0048] Step 2: Put the catalyst-based carbon fiber cloth into the reactor lining of step 1, seal the polytetrafluoroethylene lining into a stainless steel mold, place it in a constant temperature drying oven under closed conditions, and heat it at 100°C for reaction 10h.
[0049] Step 3: After the reaction is completed, cool down to room temperature with the furnace, then take out the carbon fiber cloth, wash it with distilled water and absolute ethanol in sequence, and place the washed carbon fiber in a vacuum drying oven and dry it in vacuum at 40°C for 24 hours. An array structure of nickel hydroxide is obtained.
[0050] Step 4: Put the precursor prepared in Step 3 in a tube furnace, add phosphorus source sodium hypophosphite, and react at 300°C for...
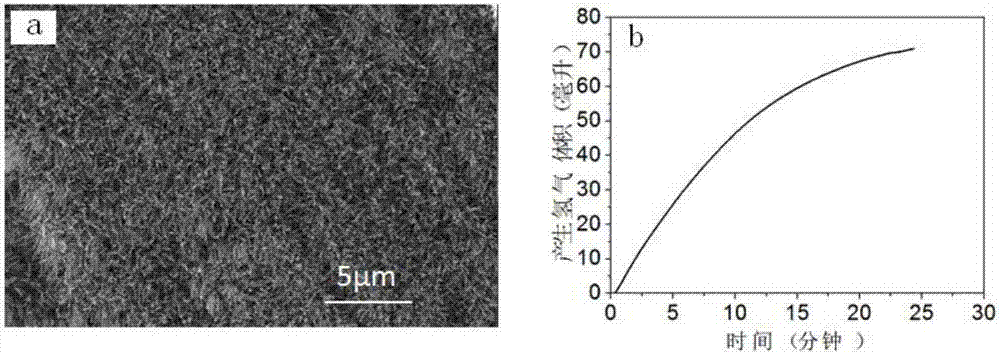
example 3
[0053] Step 1: Add 90mL of distilled water to the PTFE liner, add 1.21g of ferric nitrate, 1.873g of cobalt nitrate, 10.6g of ammonium fluoride and 1.6g of urea in each milliliter of water and stir until the solids are completely dissolved and form a transparent solution.
[0054] Step 2: Put the catalyst substrate titanium sheet into the reaction kettle lining of step 1, seal the polytetrafluoroethylene lining into a stainless steel mold, place it in a constant temperature drying oven under closed conditions, and heat it at 120°C for reaction 6h.
[0055] Step 3: After the reaction is completed, cool down to room temperature with the furnace, then take out the titanium sheet, wash it with distilled water and absolute ethanol in sequence, and place the washed titanium mesh in a vacuum drying oven and vacuum dry it at 40°C for 24 hours , to obtain the array structure of iron-cobalt hydroxide.
[0056] Step 4: Put the precursor prepared in Step 3 in a tube furnace and add phos...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com