Pharmaceutical salt of EGFR inhibitor and crystal form, preparation method and application of pharmaceutical salt
A technology of inhibitors and medicinal salts, applied in the field of medicine, can solve the problems of lack of research and protection of medicinal forms of raw materials, meet the requirements of bioavailability and drug efficacy, high solubility and stability, and facilitate processing and operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0070] The preparation method of the crystal form of the pharmaceutically acceptable salt of the EGFR inhibitor comprises:
[0071] The EGFR inhibitor in the form of free base and an acid form a salt in alcohol solvent or water, and then add (preferably dropwise) a ketone solvent for crystallization to obtain the crystalline form of the pharmaceutically acceptable salt of the EGFR inhibitor. The EGFR inhibitor in free base form and the acid preferably form equimolar salts.
[0072] Preferably, the alcoholic solvent has 1-6 carbon atoms; more preferably, the alcoholic solvent is methanol or ethanol.
[0073]Preferably, in the above preparation method, the amount ratio of the alcohol solvent to the EGFR inhibitor is 2-5ml:1g, preferably 3ml:1g.
[0074] Preferably, in the above preparation method, the ketone solvent has 3-6 carbon atoms; more preferably, the ketone solvent is acetone.
[0075] Preferably, in the above preparation method, the dosage ratio of the ketone solvent ...
Embodiment 1
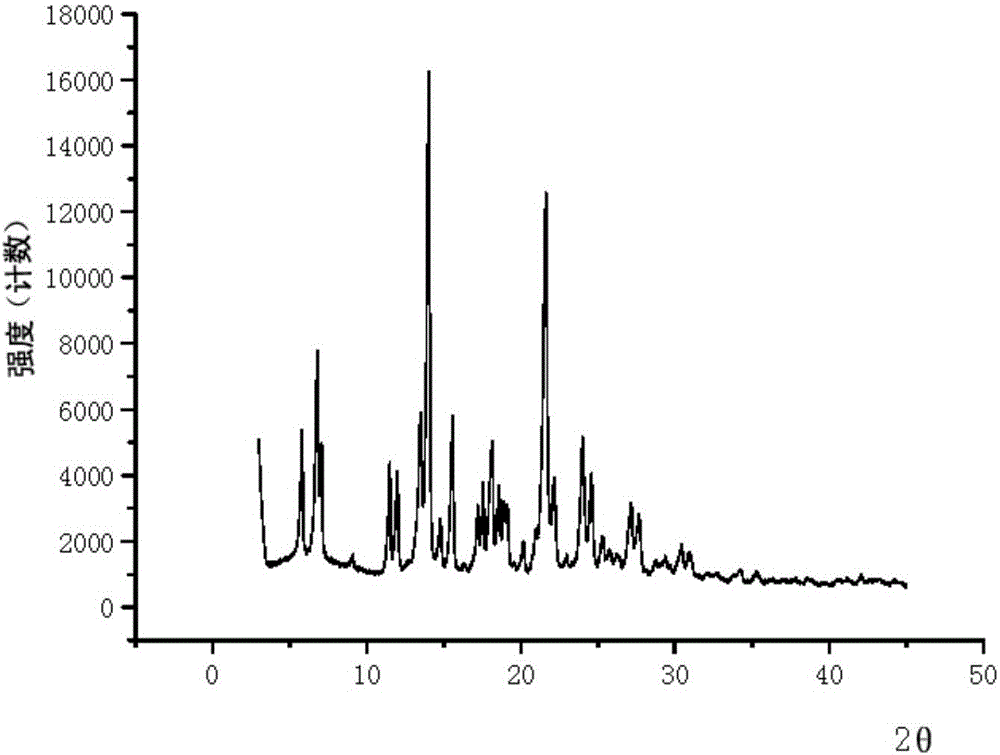
[0088] Example 1: EGFR inhibitor mesylate (form A).
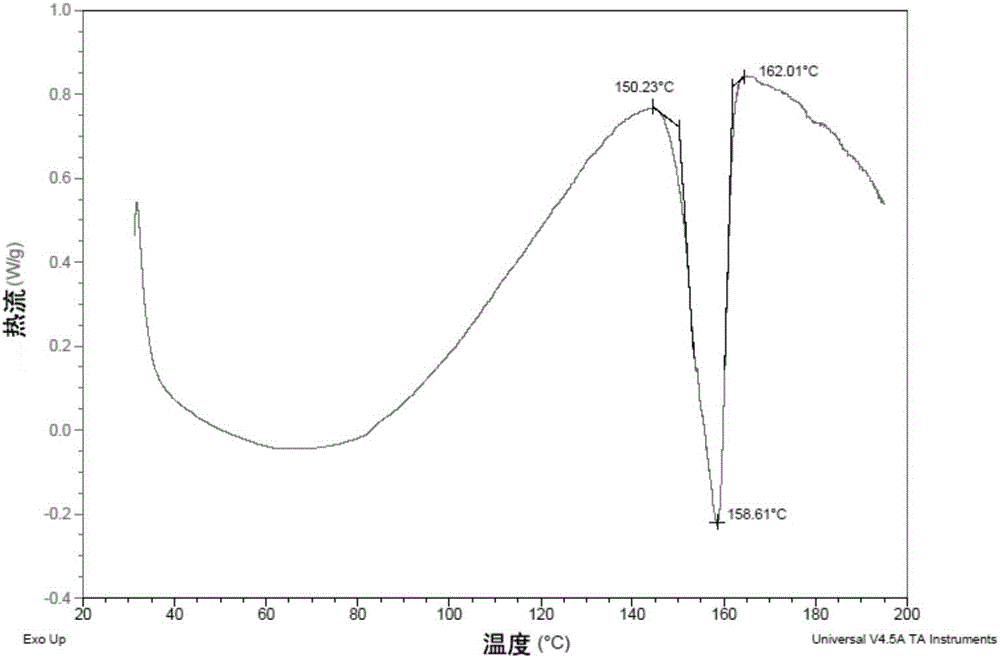
[0089] At room temperature, weigh the EGFR inhibitor (12.0 g, 24 mmol) in the form of free base, put it in a 500 mL single-necked bottle, and add 36 mL of ethanol. Add methanesulfonic acid (2.32g, 24mmol), stir and dissolve at 40-50°C. Then 240 mL of acetone was added dropwise, stirred at 0-5°C, and a gray solid was precipitated. Suction filtration, rinse the filter cake with a small amount of acetone, and dry in a vacuum oven at 60°C to obtain a light brown solid (10.1g, purity 99.0%, moisture 0.97%, melting point 150.5-152.8°C).
[0090] The structural confirmation data of the above products are as follows:
[0091] 1 H-NMR (400MHz, DMSO-d 6 ):δ9.47(1H,s),9.39(1H,s),9.30(1H,s),9.15(1H,s),8.62(1H,s),8.56~8.55(1H,d),8.48~ 8.47(1H,d),8.25~8.24(1H,d),8.06~8.04(1H,d),7.76(1H,s),7.33~7.30(1H,m),7.06(1H,s),6.83~ 6.79(1H,dd),6.46~6.41(1H,d),5.89~5.86(1H,d),4.00(3H,s),3.95(3H,s),3.27(4H,m),2.84(6H, s), 2.64(3H,s), 2.29(3H,s...
Embodiment 2
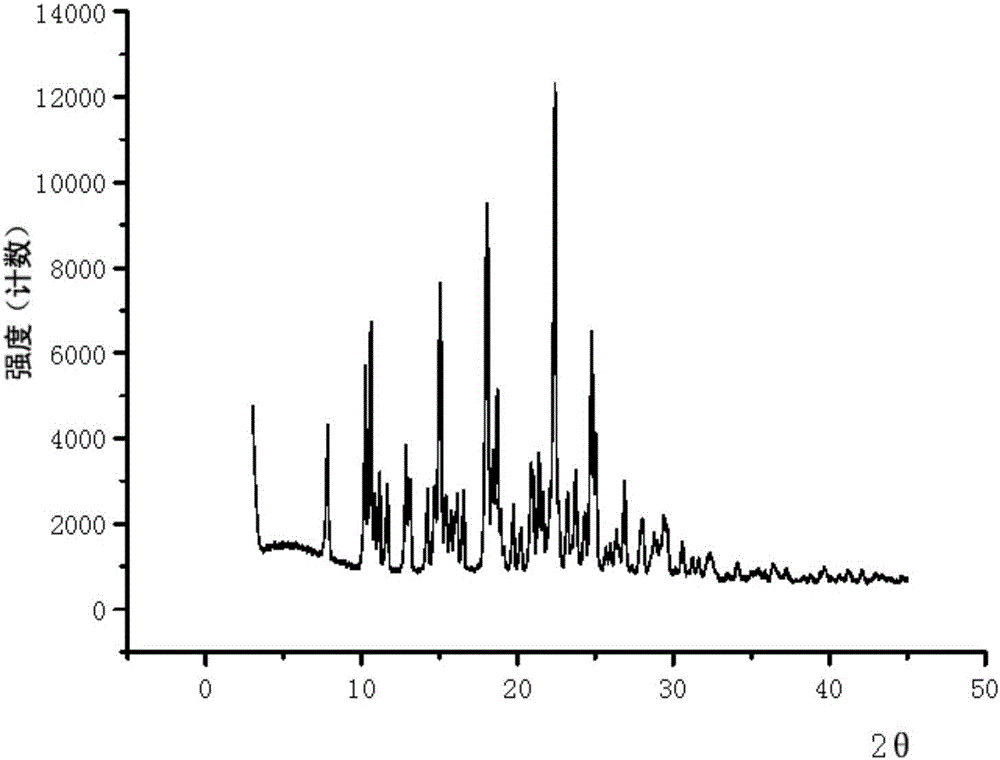
[0097] Example 2: EGFR inhibitor p-toluenesulfonate (form B).
[0098] At room temperature, weigh the EGFR inhibitor (9.0 g, 18 mmol) in the form of free base, put it in a 500 mL single-necked bottle, and add 27 mL of ethanol. Add p-toluenesulfonic acid monohydrate (4.56g, 18mmol), stir and dissolve at 40-50°C. Then 240 mL of acetone was added dropwise, and a gray solid was precipitated. After suction filtration, the filter cake was rinsed with a small amount of acetone and dried to obtain a light brown solid (10.1 g, purity 98.7%, moisture 0.57%, melting point 240.1-243.2°C).
[0099] The structural confirmation data of the above products are as follows:
[0100] 1 H-NMR (400MHz, DMSO-d 6 ):δ9.47(1H,s),9.37(1H,s),9.24(1H,s),9.14(1H,s),8.56~8.55(1H,d),8.48~8.47(1H,d), 8.24~8.23(1H,d),8.07~8.05(1H,d),7.77(1H,s),7.50~7.49(2H,d),7.30~7.29(1H,m),7.06(1H,s), 6.80~6.76(1H,dd),6.45~6.41(1H,d),5.88~5.86(1H,d),4.00(3H,s),3.95(3H,s),3.27(4H,m),2.84( 3H, s), 2.64(3H, s), 2.29(3H, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com