Preparation method of tryptanthrin compound
The technology of a compound, tryptanthrin, is applied in the field of preparation of tryptanthrin compounds, which can solve the problems of harsh conditions, heavy pollution and high cost, and achieve the effect of mild conditions, simple raw materials and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
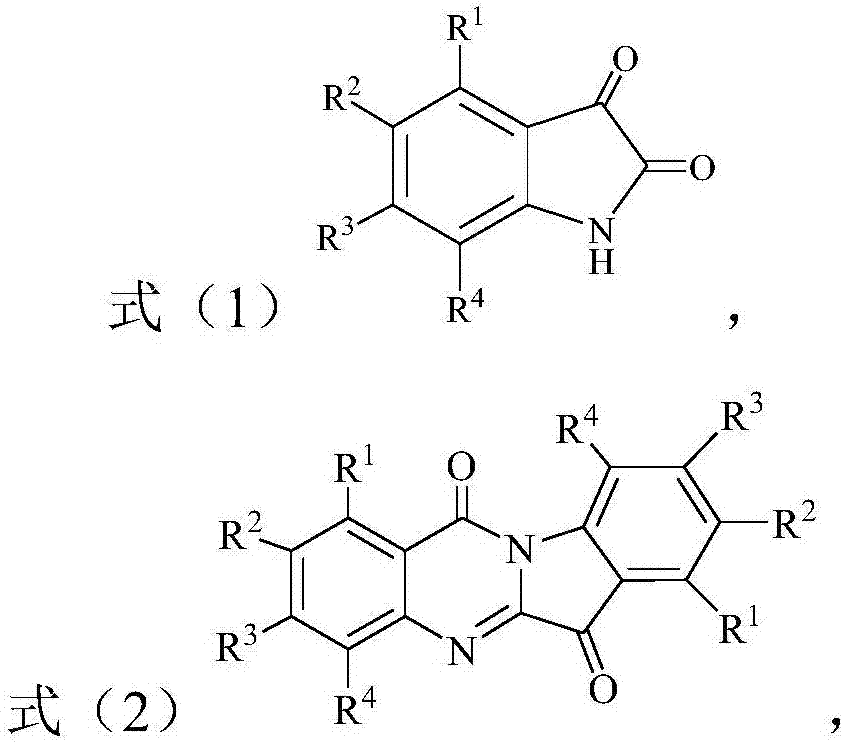
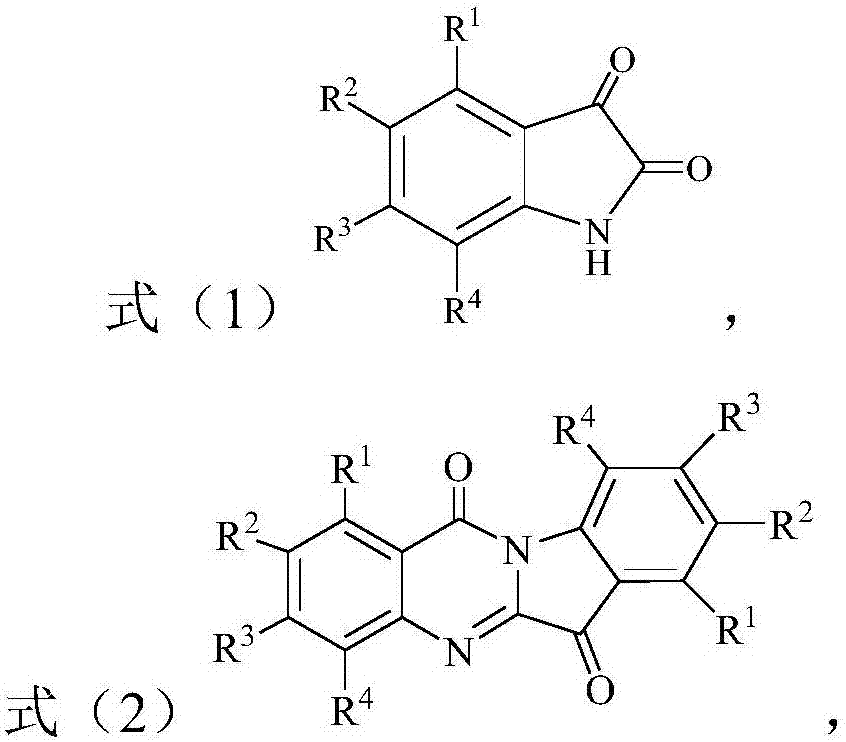
[0013] The present invention provides a preparation method of tryptanthrin compound, said tryptanthrin compound is a compound of the structure shown in formula (2), the method comprising: in the presence of peroxide and basic compound, in In an organic solvent, the compound of the structure shown in the formula (1) is subjected to an oxidative cyclization reaction to obtain a compound of the structure shown in the formula (2); the peroxide is selected from tert-butyl hydroperoxide and / or hydrogen peroxide ;
[0014]
[0015] Among them, R 1 -R 4 Each is independently selected from H, C1-C10 alkyl, C1-C10 alkoxy and halogen.
[0016] According to the present invention, the method can carry out the oxidative cyclization of the compound of the structure shown in the above formula (1) in the presence of tert-butyl hydroperoxide and a basic compound, thereby preparing the compound of the structure shown in the formula (2) .
[0017] Among them, specific examples of C1-C10 al...
Embodiment 1
[0051] This example is used to illustrate the preparation method of tryptanthrin compounds of the present invention.
[0052]
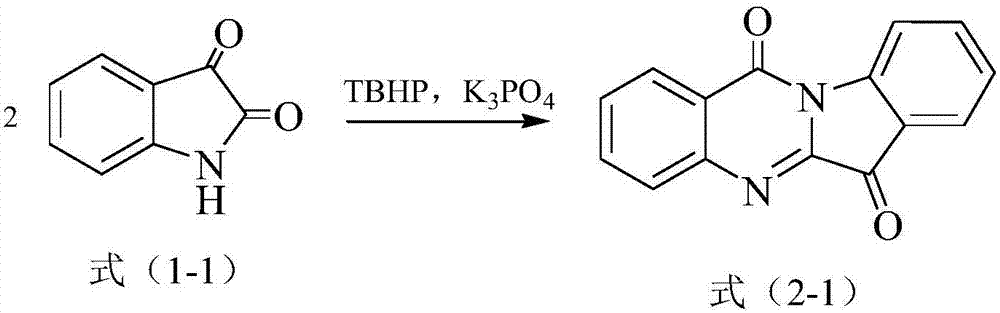
[0053] The compound of the structure shown in 1mmol formula (1-1), 65% by weight of TBHP aqueous solution (consumption makes the amount of TBHP be 1mmol) and 1mmol of K 3 PO 4 Added into 4mL of acetonitrile, reacted at 25°C and a stirring speed of 600rpm for 12h, extracted with dichloromethane, concentrated and used silica gel column chromatography (dichloromethane / petroleum ether mixture with a volume ratio of 3:2 was used as eluate) was separated and purified to obtain the compound of formula (2-1) (0.45 mmol, yield 90%).
[0054] 1 H NMR (600MHz, CDCl 3 ): δ=8.61(d, J=7.8Hz, 1H), 8.42(d, J=7.8Hz, 1H), 8.02(d, J=7.8Hz, 1H), 7.91(d, J=6.6Hz, 1H ),7.85(d,J=7.2Hz,1H),7.78(d,J=7.2Hz,1H),7.67(d,J=7.2Hz,1H)7.43(d,J=7.2Hz,1H).
[0055] 13 C NMR (150MHz, CDCl 3 ): δ=182.5, 158.0, 146.5, 146.3, 144.3, 138.2, 135.1, 130.7, 130.2, 127.5, 127.2, 125.4...
Embodiment 2
[0057] This example is used to illustrate the preparation method of tryptanthrin compounds of the present invention.
[0058]
[0059] According to the method described in Example 1, the difference is that the compound of the structure shown in the formula (1-2) is used to replace the compound of the structure shown in the formula (1-1), and K 2 CO 3 instead of K 3 PO 4 , thereby obtaining the compound (0.43 mmol, yield 86%) represented by formula (2-2).
[0060] 1 H NMR (400MHz, CDCl 3 ): δ=8.35(d, J=8.0Hz, 1H), 8.09(s, 1H), 7.82(d, J=8.4Hz, 1H), 7.61(s, 1H), 7.58(d, J=8.4Hz ,1H),7.48(d,J=8.0Hz,1H),2.51(s,3H),2.41(s,3H).
[0061] 13 C NMR (100MHz, CDCl 3 ): δ=182.2, 157.4, 144.1, 143.8, 143.6, 140.8, 138.4, 137.0, 136.0, 130.2, 126.9, 125.1, 123.2, 121.8, 117.4, 21.7, 21.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com