Heterocyclic compound for treating Alzheimer disease and preparation method thereof
A technology of heterocyclic compounds and solvates, applied in the field of medicine, can solve the problems of serious adverse reactions of liver toxicity and few clinical problems, and achieve the effects of good acetylcholinesterase activity, inhibiting acetylcholinesterase activity and improving memory function.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
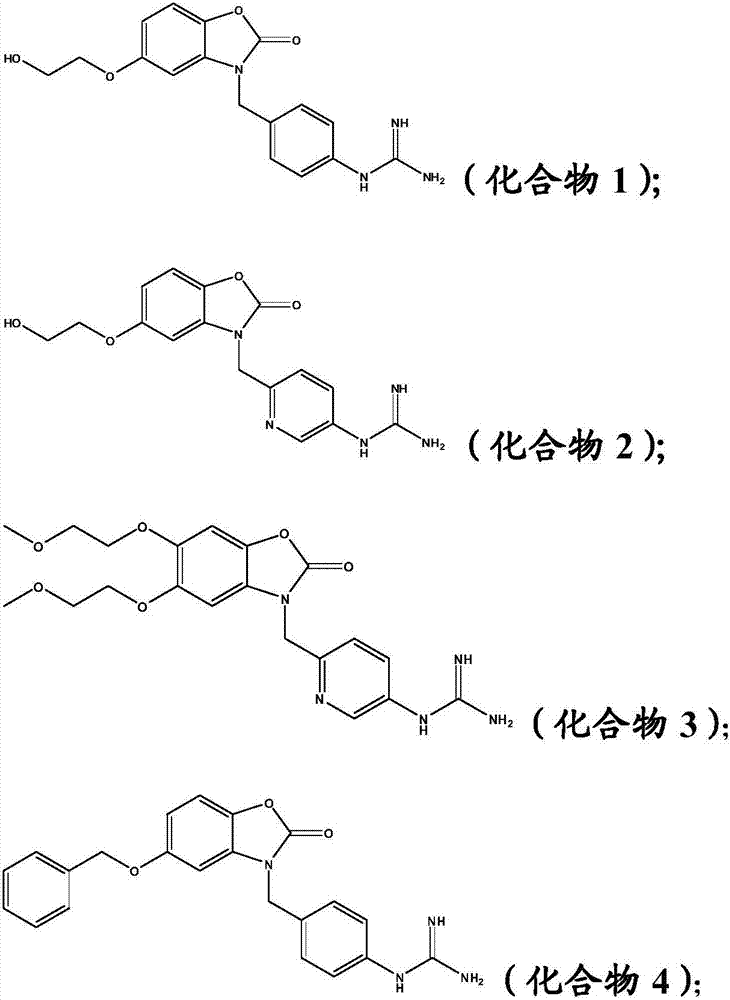
[0053] Example 1: 1-(4-((5-(2-hydroxyethoxy)-2-oxobenzo[d]oxazol-3(2H)-yl)methyl)phenyl)guanidine (compound 1)
[0054]
[0055] 5-(2-hydroxyethoxy)benzo[d]oxazol-2(3H)-one (3.9g), 1-(4-(bromomethyl)phenyl)guanidine (5.0g), carbonic acid A mixture of potassium (5.6g) in acetonitrile (400mL) was stirred and refluxed for 12h, the solvent was removed in vacuo, and the residue was eluted by gradient chromatography on a silica gel column. The mobile phase was petroleum ether / ethyl acetate 10:1 to 1:1, and the collected According to the corresponding components, 6.1 g of the title compound was obtained as a white solid, with a yield of 89.6% and a content of 99.1%.
[0056] ESI-MS: 343.13[M+H] +
[0057] Elemental analysis: theoretical value / measured value, C(59.64 / 59.54), H(5.30 / 5.38), N(16.37 / 16.48), O(18.69 / 18.71)
[0058] 1 H NMR (400MHz, CDCl 3 )δ8.99(s,1H),7.86(s,1H),7.34(s,1H),7.15(d,1H),6.91(d,2H),6.74(d,1H),6.64(s,2H ), 6.32(d,2H), 4.93(s,1H), 4.74(s,2H), 4.31(t,2...
Embodiment 2
[0059] Example 2: 1-(6-((5-(2-hydroxyethoxy)-2-oxobenzo[d]oxazol-3(2H)-yl)methyl)pyridin-3-yl) Guanidine (compound 2)
[0060]
[0061] 5-(2-hydroxyethoxy)benzo[d]oxazol-2(3H)-one (3.9g), 1-(6-(bromomethyl)pyridin-3-yl)guanidine (5.0g ), a mixture of potassium carbonate (5.6g) in acetonitrile (400mL) was stirred and refluxed for 12h, the solvent was removed in vacuo, and the residue was eluted by gradient chromatography on a silica gel column, and the mobile phase was petroleum ether / ethyl acetate 8:1 to 1: 1. Collect the corresponding components to obtain 5.9 g of the title compound as a white solid, with a yield of 85.4% and a content of 99.2%.
[0062] ESI-MS: 344.13[M+H] +
[0063] Elemental analysis: theoretical value / measured value, C(55.97 / 55.84), H(4.99 / 4.88), N(20.40 / 20.52), O(18.64 / 18.76)
[0064] 1 H NMR (400MHz, CDCl 3 )δ8.98(s,1H),7.86(s,1H),7.72(d,1H),7.56(d,1H),7.34(s,1H),7.26(d,1H),7.13(d,1H ), 6.74(d,1H), 6.64(s,2H), 4.93(s,1H), 4.54(s,2H), 4.31(t,2H...
Embodiment 3
[0065] Example 3: 1-(6-((5,6-bis(2-methoxyethoxy)-2-oxobenzo[d]oxazol-3(2H)-yl)methyl)pyridine -3-yl)guanidine (compound 3)
[0066]
[0067] 5,6-bis(2-methoxyethoxy)benzo[d]oxazol-2(3H)-one (5.7g), 1-(6-(bromomethyl)pyridin-3-yl ) The mixture of guanidine (5.0g), potassium carbonate (5.6g) in acetonitrile (400mL) was stirred and refluxed for 16h, the solvent was removed in vacuo, and the residue was eluted by silica gel column chromatography gradient, and the mobile phase was ether / ethyl acetate 5: From 1 to 1:1, the corresponding components were collected to obtain 7.0 g of the title compound as a light yellow solid, with a yield of 81.4% and a content of 98.9%.
[0068] ESI-MS: 432.18[M+H] +
[0069] Elemental analysis: theoretical value / measured value, C(55.68 / 55.75), H(5.84 / 5.88), N(16.23 / 16.14), O(22.25 / 22.23)
[0070] 1 H NMR (400MHz, CDCl 3 )δ8.98(s,1H),7.86(s,1H),7.73(d,1H),7.54(d,1H),7.22(s,1H),7.13(d,1H),6.74(s,1H ), 6.64(s,2H), 4.50(s,2H), 4.31(t,4H), 3.7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com