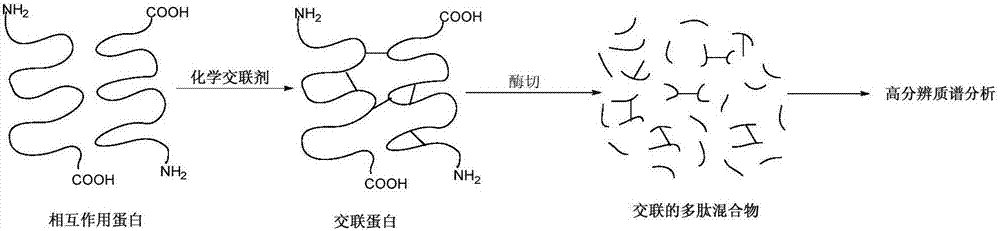
Multifunctional chemical crosslinking agent, preparation method and application thereof
A chemical cross-linking agent, multifunctional technology, applied in the field of protein and its function research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1: Synthesis of Leiker 1
[0063] (1) Synthesis of compound 2
[0064]
[0065] Compound 1 (23.2 mg, 0.1 mmol), sulfo-NHS (71.6 mg, 0.33 mmol) and EDCI (67.1 mg, 0.35 mmol) were dissolved in anhydrous DMSO (5 mL), and the reaction mixture was stirred at room temperature for 24 hours. Then 40 mL of anhydrous THF was added, the solution became turbid, and after standing for 12 hours, the viscous solid settled at the bottom of the round bottom flask and the solvent became clear. Carefully pour off the supernatant, and then wash the viscous solid twice with 5 mL of anhydrous THF, and finally obtain the white viscous crude product 2, which is directly used in the next reaction without further purification;
[0066] (2) Synthesis of compound 4
[0067]
[0068] Dissolve compound 3 (500mg, 3.6mmol) in anhydrous DMF (7.5mL), then add anhydrous potassium carbonate (745mg, 5.4mmol), stir at 30°C for 30 minutes, and then slowly add 3-bromopropyne (462 μL, 5.4 mmol...
Embodiment 2
[0096] Embodiment 2: the synthesis of Leiker 2
[0097] (1) Synthesis of Compound 11
[0098]
[0099] Compound 7 (69.2 mg, 0.181 mmol) obtained in Example 1 was dissolved in DMF / DCM / H 2 O (2mL / 2mL / 2mL), followed by adding azide biotin 10 (77mg, 0.236mmol), CuSO 4 ·5H 2 O (2.3mg, 0.009mmol) and sodium ascorbate (5.4mg, 0.027mmol), the reaction mixture was stirred at room temperature for 24 hours. After the reaction, 4 mL of water was added, and extracted three times with ethyl acetate (12 mL×3). The combined organic phases were washed with saturated brine (5 mL), and finally washed with anhydrous Na 2 SO 4 Dry, filter, and concentrate the filtrate under reduced pressure. The residue was purified by flash silica gel column chromatography (6%~10% methanol in dichloromethane solution) to give bright yellow solid 11 (117mg, 91%);
[0100] The characterization data of the obtained compound 11 are as follows:
[0101] 1 H NMR (400MHz Methanol-d 4 ): δ1.44(m, 2H), 1.48(s...
Embodiment 3
[0118] Embodiment 3: the synthesis of Leiker 3
[0119] (1) Synthesis of compound 15
[0120]
[0121] 3-Amino-1-propanol (300mg, 4mmol) was dissolved in anhydrous dichloromethane (15mL), the reaction solution was placed in an ice bath, and a dichloromethane solution (12mL) of FmocCl (528mg, 2mmol) was added dropwise to The reaction solution lasted for 30 minutes. The temperature was raised to room temperature and stirring was continued for 1.5 hours. Wash three times with 0.5M HCl solution (5mL×3), combine the organic phase and wash with saturated brine (5mL), and finally wash with anhydrous Na 2 SO 4 Dry, filter, and concentrate the filtrate under reduced pressure. The residue was purified by flash silica gel column chromatography (50% ethyl acetate petroleum ether solution) to give white solid 15 (585 mg, 98%);
[0122] (2) Synthesis of compound 16
[0123]
[0124] p-Nitrophenol (211mg, 1.52mmol) was dissolved in anhydrous THF (37mL), then compound 15 (519mg, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com