Sodium channel blockers
A substituted and unsubstituted technology, applied in the field of sodium channel blockers, can solve problems such as sedation problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
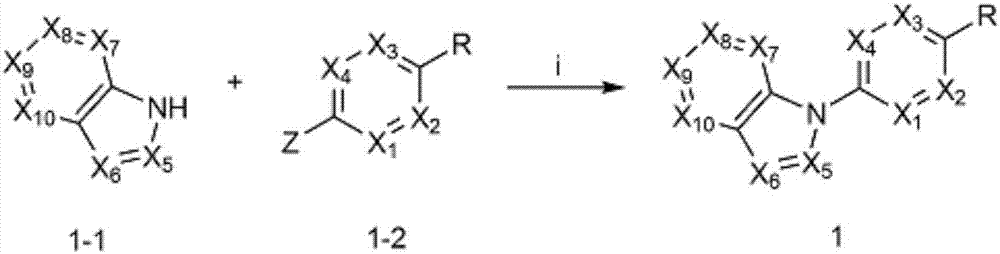
Image
Examples
Embodiment 1
[0438] Example 1) Preparation of (5-chloro-4-(5-chloro-3-methyl-1H-indazol-1-yl)-2-fluoro-N-(methylsulfonyl)benzamide
[0439]
[0440] Dissolve 0.2g (1.2mmol) of 5-chloro-3-methyl-1H-indazole in 10mL of anhydrous N,N-dimethylformamide, add 0.78g (2.4mmol) of cesium carbonate and 0.32g (1.2 mmol) 5-Chloro-2,4-difluoro-N-(methylsulfonyl)benzamide. The reaction mixture was stirred at 150 °C for 1 hour, the organic layer was separated, treated with magnesium sulfate, filtered, and concentrated under reduced pressure. The residue was separated by column chromatography to obtain 0.15 g (yield: 30%) of the title compound.
[0441] 1H NMR (MeOD) δ: 7.84 (s, 1H), 7.82-7.81 (d, 1H), 7.45-7.43 (d, 2H), 7.30-7.28 (d, 1H), 3.30 (s, 3H), 2.60 ( s, 3H).
Embodiment 6
[0442] Example 6) Preparation of 4-(3-acetylamino-5-chloro-1H-indazol-1-yl)-5-chloro-2-fluoro-N-(methylsulfonyl)benzamide
[0443]
[0444] Dissolve 0.1g (0.24mmol) of 4-(3-amino-5-chloro-1H-indazol-1-yl)-5-chloro-2-fluoro-N-(methylsulfonyl)benzamide in 5mL without In water tetrahydrofuran, 0.018 g (0.24 mmol) of acetyl chloride was added thereto. The reaction mixture was stirred at room temperature for 2 hours, the organic layer was separated, treated with magnesium sulfate, filtered, and concentrated under reduced pressure. The residue was separated by column chromatography to obtain 0.033 g (yield: 30%) of the title compound.
[0445] 1H NMR (MeOD) δ: 7.80-7.99(d, 1H), 7.64-7.62(d, 1H), 7.40-7.38(d, 1H), 7.28-7.27(d, 2H), 3.15(s, 3H), 2.25(s, 3H).
Embodiment 24
[0446] Example 24) Preparation of 5-chloro-4-(3-chloro-5-cyclopropyl-1H-indazol-1-yl)-2-fluoro-N-(methylsulfonyl)benzamide
[0447]
[0448] Under nitrogen, 25 mL of tetrahydrofuran was added to 1.0 g (7.0 mmol) of zinc(II) chloride, and 4.0 mL (7.0 mmol, 1.7 M of tetrahydrofuran) of cyclopropylmagnesium bromide was added thereto, followed by stirring at room temperature for 30 min . Under nitrogen, the reaction mixture was added to 0.4 g (1.72 mmol) of 5-bromo-3-chloro-1H-indazole, and then reacted at 100° C. for 10 minutes using a microwave reactor. The organic layer was separated and concentrated under reduced pressure. The residue was separated by column chromatography to obtain 0.24 g (yield: 80%) of 3-chloro-5-cyclopropyl-1H-indazole.
[0449] 1H NMR (MeOD) δ: 7.36 (d, 1H), 7.29 (s, 1H), 7.18 (d, 1H), 2.00 (m, 1H), 0.95 (d, 2H), 0.68 (d, 2H).
[0450] 0.10 g (yield: 18%) of the title compound were prepared in the same manner as described in Example 1, except that 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com