Compound for enhancing coupling degree of complex TRPV4-KCa2.3 and application thereof in resisting hypertension
A technology of trpv4-kca2.3 and compounds, applied in the field of antihypertensive drugs, can solve problems such as unclear interaction sites
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1: the preparation method of compound
[0058] (1) Dissolve compound 1-propylenediamine (30g, 405mmol, 1eq) in 150mL of dichloromethane, stir in an ice bath; dissolve and dilute 2-tert-butyl sodium bicarbonate (16.1g, 73mmol, 0.18eq) in 50mL of dichloromethane; slowly pour the mixture into a flask, and stir at room temperature for 3 hours; after the reaction is complete, use thin-layer chromatography (TLC) to detect, dilute with 50mL of dichloromethane and wash with water several times, then wash with saturated NaCl solution Wash, followed by anhydrous Na 2 SO 4 After drying and concentrating, Compound 2 (19g, 27%) was obtained;
[0059] (2) Compound 2 (10g, 57mmol, 1eq) and triethylamine (TEA) (8.7g, 86mmol, 1.5eq) were dissolved in 100mL of dichloromethane and stirred in an ice bath; diphenylacetyl chloride (13.1g, 57mmol ,1eq) was dissolved in 30mL of dichloromethane, and slowly poured into the flask, and stirred at room temperature for 2.5 hours; after...
Embodiment 2
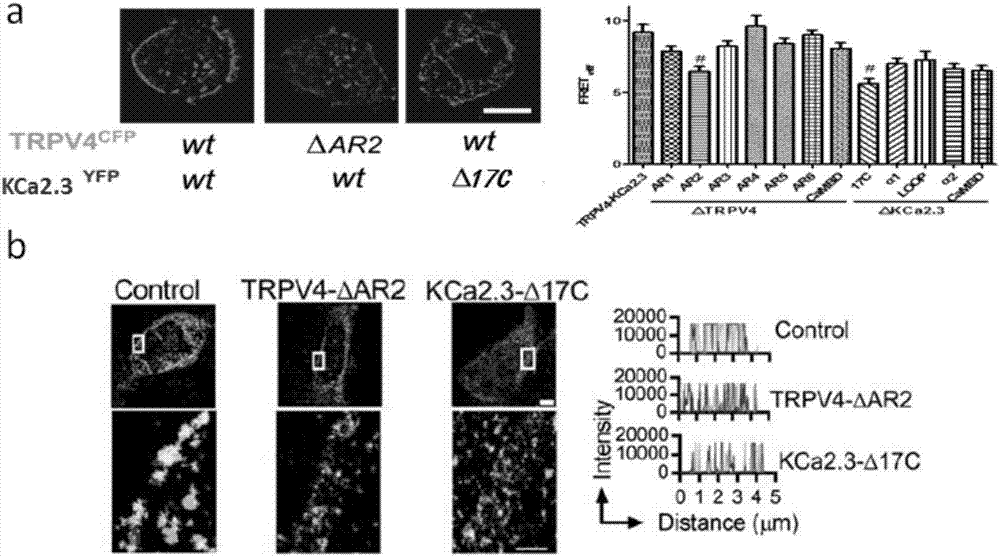
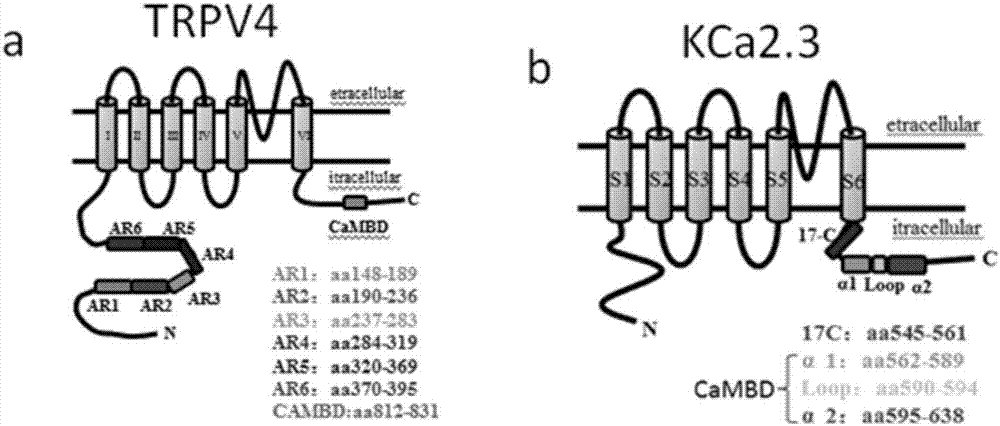
[0062] Example 2: Find the structural domain of the interaction site between TRPV4 protein and KCa2.3 protein
[0063] Experimental method: select possible binding sites according to the three-dimensional structure and functional characteristics of TRPV4 protein and KCa2.3 protein (such as figure 1 shown), the selected domains are mainly used to regulate the relationship between protein-protein, which is a platform for protein interaction. The selected binding site was mutated to delete the binding site, and the primers used are shown in Table 1.
[0064] Table 1
[0065]
[0066]
[0067] The PCR reaction system used is: template (CFP-TRPV4 whole gene or YFP-KCa2.3 whole gene) 0.5 μl, PrimStar HS 25 μl, upstream and downstream primers 0.5 μl each, add H 2 O to make up to 50 μl. The PCR reaction program was: pre-denaturation at 95°C for 2min, denaturation at 95°C for 30s, annealing at 55°C for 30s, extension at 68°C for 5min, 30 cycles, and full extension at 68°C for ...
Embodiment 3
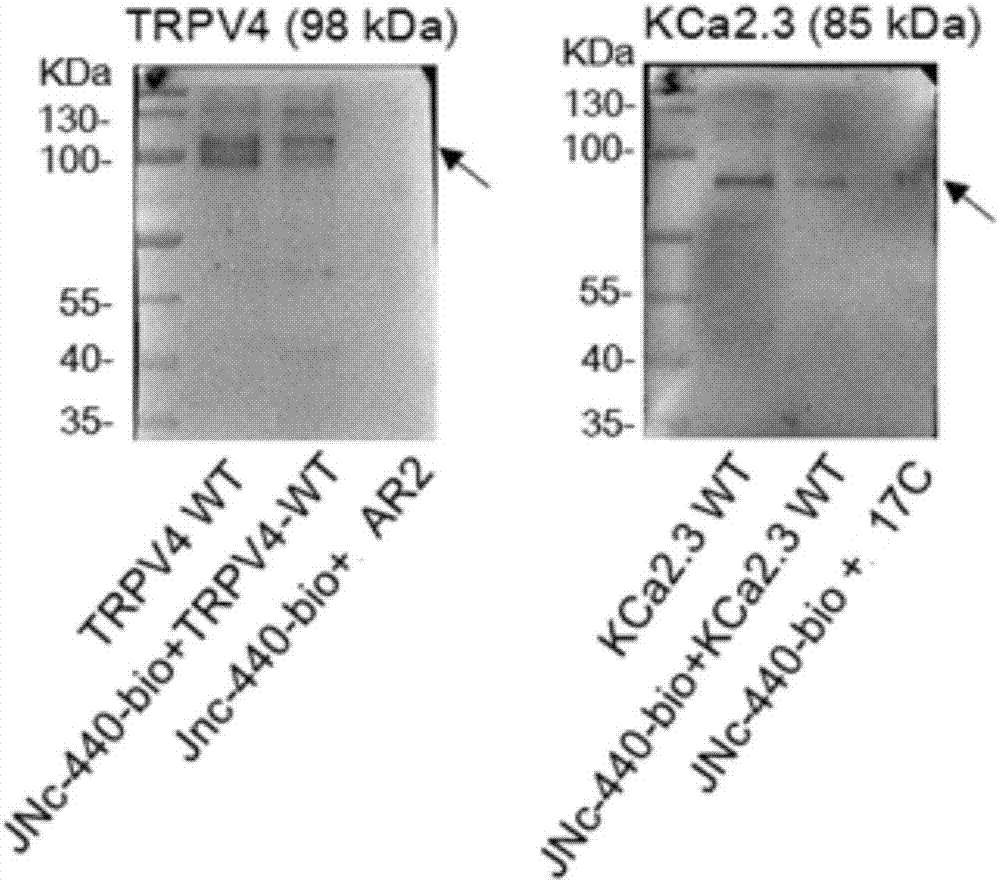
[0073] Example 3: The compound prepared in Example 1 targets the domain AR2 of TRPV4 and the domain 17C of KCa2.3.
[0074] experimental method:
[0075] The primary isolated C57BL / 6J mouse mesenteric endothelial cells were cultured in a cell constant temperature incubator, and biotinylated JNc-440 (10 μM / L) or short peptide and biotinylated JNc-440 (10 μM / L) were added to the cells. Incubate for 96 hours. The cell protein was lysed with RIPA, and the protein supernatant was added to 10 μL streptavidin-coated magnetic beads, and incubated overnight at 4°C. Adsorb the incubated suspension with a magnetic stand to obtain an avidinylated magnetic bead-biotinylated JNc-440-protein complex. Resuspend the complex with 50 μL 1xLoadingbuffer and boil in a boiling water bath for 5 minutes. The samples were separated by SDS-PAGE, the protein was transferred to PVDF membrane, blocked with 5% BSA at room temperature for 4 hours, the primary antibody was incubated overnight at 4°C, the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com