STie2 fusion protein, carrier thereof and pharmaceutical composition containing sTie2 fusion protein
A technology of fusion protein and stie1-c, which is applied in the field of genetic engineering and protein engineering, can solve the problems of unclear mechanism and failure to predict thrombus formation, and achieve the effect of inhibition and promotion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1: Recombinant Construction of Fusion Protein Expression Plasmids
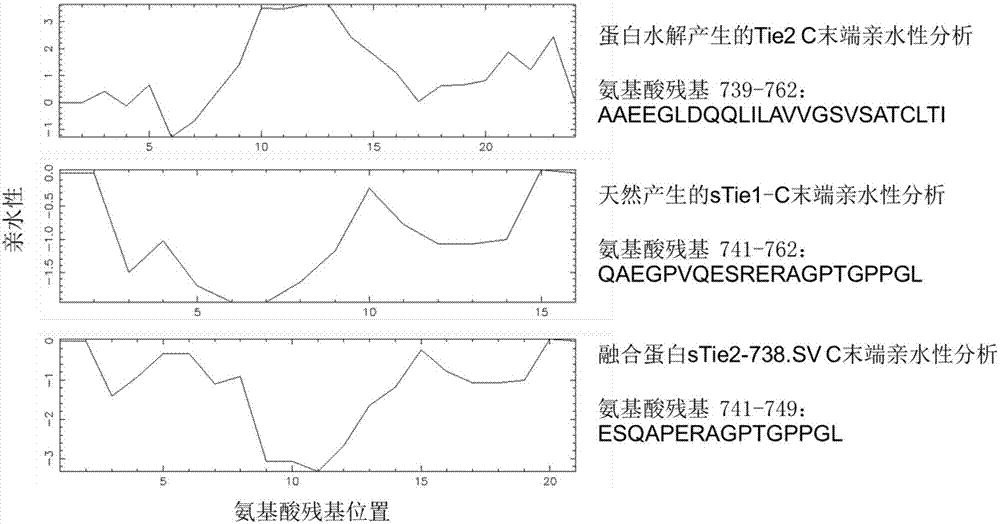
[0066] The present invention carries out genetic engineering transformation on the basis of the human source Tie2 gene sequence (GenBank: NM_000459) template, and designs 5 kinds of partial expression sequences of the Tie2 gene containing different functional regions ( figure 2 ). 1. Tie2-781: Contains 1-781 amino acids of the Tie2 gene, has a transmembrane region, and does not have extracellular secretion ability. 2. sTie2-751.6His: contains amino acids 1-751 of Tie2 gene, without transmembrane region. Six His amino acid tags were added to the C-terminus. A soluble sTie2 mimic formed by proteolysis. 3. sTie2-738.SV.6His: Contains amino acids 1-738 of the Tie2 gene, this fragment is compatible with the extracellular binding region before the sTie1-C terminal sequence of soluble sTie1, and does not contain the transmembrane region. 11 amino acid sequences of sTie1-C and 6 His amino acid tags...
Embodiment 2
[0068] Example 2: Effect of C-terminal amino acid composition expressed in different cells on soluble protein expression and extracellular secretion
[0069] The cultured cells were transfected with 5 kinds of plasmid DNAs respectively containing Tie2-781, sTie2-738.6His, sTie2-738.SV.6His, sTie2-738.Fc and sTie2-544.SV.6His gene expression sequences, and determined by ELISA The content of Tie2 protein in cell culture supernatant and cell lysate was determined by method, and the effect of sTie1-C terminal on the yield and secretion of fusion protein was compared. The specific method is: inoculate 2.4x10^4 cells in a 6-well plate. Mix 2.5 μg with lipefectamine cell transfection reagent (Life Technology), add to the cultured cells, mix well, after 24 hours of transfection, replace into DMEM culture medium containing 1% FBS, incubate for 24 hours, take the supernatant, and collect the cells , the content of Tie2 in the sample was measured by ELISA quantitative assay method, and ...
Embodiment 3
[0071] Embodiment 3: Culture cell HEK293T produces fusion protein and Ni-NTA chromatographic column purification method
[0072] Collection of fusion protein: HEK293T cells were cultured with DMEM medium (HyClone) containing 10% FBS, and when the cell density grew to 60%-80%, fresh 10% FBS DMEM medium was replaced, and the recombinant plasmid and liposome mixture , transfected into the cells by liposome transfection, the medium was replaced with DMEM medium containing 1% FBS on the second day after transfection, and the culture was continued until the third day, and the culture medium was collected. For three consecutive days thereafter, the DMEM medium with 1% FBS was changed every day, and the supernatant of the culture solution was harvested after 24 hours of cultivation. The supernatant contains the fusion protein expressed by the cells. ELISA assay was used to identify and confirm the expression of sTie2 fusion protein.
[0073] Protein purification process: collect the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com